- Record: found
- Abstract: found
- Article: found
Characterization and drug sensitivity profiling of primary malignant mesothelioma cells from pleural effusions
Read this article at
Abstract
Background
Patients with malignant mesothelioma have a poor prognosis and only 40% respond to first line treatment; a combination of pemetrexed and cisplatin or carboplatin. We used primary malignant mesothelioma cells and an ex vivo chemosensitivity assay with future purpose to predict best choice of treatment. The clinical outcome of these patients might be predicted by measuring drug sensitivity.
Methods
Pleural effusions containing primary malignant mesothelioma cells were received from the diagnostic routine. We characterized and tested the chemosensitivity of 18 malignant samples and four benign samples from 16 different patients with pleural effusions. Cells were seeded in a 384-well plate for a robotized ex vivo testing of drug sensitivity to 32 different drugs. The primary cells were further characterized by immunocytochemistry to evaluate the proportion of malignant cells and to study the RRM1 and ERCC1 reactivity, two proteins associated with drug resistance.
Results
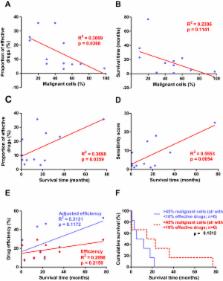
We observed great individual variability in the drug sensitivity. Primary cell isolates were affected by between one and ten drugs, and resistant to the remaining tested drugs. Actinomycin D and daunorubicin were the two drugs effective in most cases. Adjusting efficiency of individual drugs for varying proportion of tumor cells and to the average effect on benign cells correlated with effect of pemetrexed, cisplatin and survival time. General drug sensitivity, proportion of malignant cells and reactivity to RRM1 correlated to each other and to survival time of the patients.
Conclusions
The proportion of malignant cells and RRM1 reactivity in the pleural effusions correlate to drug sensitivity and survival time. The variability in response to the commonly used chemotherapies emphasizes the need for tests that indicate best individual choice of cytotoxic drugs. The efficiency of the obtained results should preferably be corrected for admixture of benign cells and effects of given drugs on benign cells.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma.
- Record: found
- Abstract: found
- Article: not found
Mesothelin: a new target for immunotherapy.
- Record: found
- Abstract: found
- Article: not found