- Record: found
- Abstract: found
- Article: not found
A practical approach for vaccinations including COVID-19 in autoimmune/autoinflammatory rheumatic diseases: a non-systematic review
Read this article at
Abstract
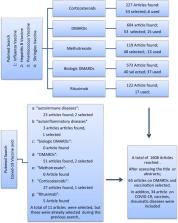
The COVID-19 pandemic has occupied the world agenda since December 2019. With no effective treatment yet, vaccination seems to be the most effective method of prevention. Recently developed vaccines have been approved for emergency use only and are currently applied to large populations. Considering both the underlying pathogenic mechanisms of autoimmune/autoinflammatory rheumatological diseases (AIIRDs) and the immunosuppressive drugs used in treatment, vaccination for COVID-19 deserves special attention in such patients. In this article, we aimed to give simple messages to the clinicians for COVID-19 vaccination in patients with AIIRDs based upon the current evidence regarding the use of other vaccines in this patient group. For this purpose, we conducted a “Pubmed search” using the following keywords: Influenza, Hepatitis B, Pneumococcal, and Shingles vaccines and the frequently used conventional and biologic disease-modifying antirheumatic drugs (DMARDs). Likewise, an additional search was performed for the COVID-19 immunization in patients with AIIRDs and considering such drugs. In summary, patients with AIIRDs should also be vaccinated against COVID-19, preferably when disease activity is under control and when there is no concurrent infection. Low-degree immunosuppression does not appear to decrease antibody responses to vaccines. Ideally, vaccinations should be done before the initiation of any biological DMARDs. Patients receiving rituximab should be vaccinated at least 4 weeks before or 6 months after treatment. Since tofacitinib may also reduce antibody responses, especially in combination with methotrexate, it may be appropriate to discontinue this drug before vaccination and to restart after 14 days of immunization.
|
Key points • COVID-19 vaccinations should preferably be made during remission in patients with autoimmune/autoinflammatory rheumatological diseases. • Low-degree immunosuppression may not interfere with antibody response to vaccines. • Ideally, vaccinations should be made before the initiation of any biological DMARDs. • Timing of vaccination is especially important in the case of rituximab. |
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: not found

