- Record: found
- Abstract: found
- Article: found
Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach
Read this article at
Abstract
Background
The fruit of Elaeagnus umbellata has high medicinal values and is an excellent source of phytochemicals. This study was aimed to evaluate the antioxidant, enzyme inhibitory and antidiabetic potential of Elaeagnus umbellata.
Methods
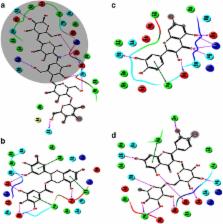
The antioxidant potential of the crude extract and subfractions of E. umbellata fruit were determined using DPPH (2, 20-diphenyl-1-picrylhydrazyl) and ABTS (2, 2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) assays. The enzyme inhibitory potentials of extracts against α-amylase and α-glucosidase enzymes were also determined. The in vivo anti-hyperglycemic effects of the extract in STZ-induced type 2 diabetes were determined using Sprague Dawley adult rats. HPLC system (Agilent 1260) was used for the identification of bioactive compounds present in extracts. Molecular docking was used to identify and compare the interaction between the compounds (active constituents) and standard inhibitor acarbose with the α-amylase and α-glucosidase active sites.
Results
The chloroform, ethyl acetate, and butanol fractions showed significant antioxidant potential with IC 50 values of 40, 45 and 60 μg/mL against DPPH and 57, 70 and 120 μg/mL against ABTS free radicals respectively. The chloroform and ethyl acetate were highly active against α-amylase and α-glucosidase (IC 50 values 58 and 200 μg/ml against α-amylase 60 and 140 μg/ml against α-glucosidase. The crude extract, chloroform, and ethyl acetate fractions were more potent in controlling the hyperglycemia in STZ-induced type 2 diabetes in rats and considerable reduction of glucose level was observed compared to the non-treated group. Furthermore, the extracts were also found useful in controlling the secondary complications associated with type 2 diabetes mellitus which was evident from the observed substantial reduction in the blood level of serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, alkaline phosphatase, total cholesterol, low-density lipoproteins, and triglycerides. The molecular docking approach indicated the favorable inhibitory interaction between the docked compounds and the active sites of the α-amylase and α-glucosidase. All docked compounds occupied the same binding site as occupied by acarbose.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Global prevalence of diabetes: estimates for the year 2000 and projections for 2030.
- Record: found
- Abstract: found
- Article: not found
Antioxidant activity applying an improved ABTS radical cation decolorization assay.
- Record: found
- Abstract: found
- Article: not found
