- Record: found
- Abstract: found
- Article: found
Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
Read this article at
Abstract
Background
Despite widespread implementation of vaccination against coronavirus disease 2019 (COVID-19), solid organ transplant recipients (SOTRs) can remain particularly vulnerable to this disease. The present study was conducted to investigate the efficacy and safety of sotrovimab in the treatment of SOTRs with COVID-19.
Methods
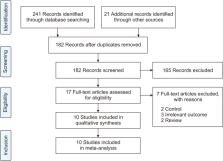
A search was performed of PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar to gather relevant evidence through July 25, 2023. The quality of the included studies was assessed using the risk of bias tool. Comprehensive Meta-Analysis software (ver. 3.0, Biostat) was employed for data analysis.
Results
Ten studies, involving a total of 1,569 patients, were included. The meta-analysis revealed significant differences between the patients administered sotrovimab and those treated with the standard of care. These differences were observed in mortality rate (odds ratio [OR], 0.15; 95% confidence interval [CI], 0.03–0.67), hospitalization rate (OR, 0.35; 95% CI, 0.21–0.57), intensive care unit (ICU) admission rate (OR, 0.16; 95% CI, 0.04–0.62), the need for supplemental oxygen therapy (OR, 0.22; 95% CI, 0.09–0.51), and the need for mechanical ventilation (OR, 0.09; 95% CI, 0.01–0.70). However, no significant difference was observed between sotrovimab and other treatments regarding the rates of hospitalization or ICU admission (P>0.05). Regarding safety, sotrovimab was associated with a lower rate of adverse events compared to the absence of sotrovimab (OR, 0.15; 95% CI, 0.02–0.86).
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: found
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement

- Record: found
- Abstract: found
- Article: found
ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions
- Record: found
- Abstract: found
- Article: not found