- Record: found
- Abstract: found
- Article: found
Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: pre-clinical study applicable in the pediatric surgical setting
Read this article at
Abstract
Objective
Mesenchymal stromal cells (MSCs) expanded in vitro have been proposed as a potential therapy for congenital or acquired skin defects in pediatrics. The aim of this pre-clinical study was to investigate the effects of intradermal injections of MSC in experimental cutaneous wound repair comparing allogeneic and autologous adipose stem cells (ASCs) and autologous bone marrow-mesenchymal stromal cells (BM-MSCs).
Methods
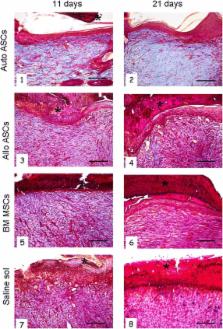
Mesenchymal stromal cells were in vitro expanded from adipose and BM tissues of young female New Zealand rabbits. MSCs were characterized for plastic adhesion, surface markers, proliferation and differentiation capacity. When an adequate number of cells (ASCs 10 × 10 6 and BM-MSCs 3 × 10 6, because of their low rate of proliferation) was reached, two skin wounds were surgically induced in each animal. The first was topically treated with cell infusions, the second was used as a control. The intradermal inoculation included autologous or allogeneic ASCs or autologous BM-MSCs. For histological examination, animals were sacrificed and wounds were harvested after 11 and 21 days of treatment.
Results
Rabbit ASCs were isolated and expanded in vitro with relative abundance, cells expressed typical surface markers (CD49e, CD90 and CD29). Topically, ASC inoculation provided more rapid wound healing than BM-MSCs and controls. Improved re-epithelization, reduced inflammatory infiltration and increased collagen deposition were observed in biopsies from wounds treated with ASCs, with the best result in the autologous setting. ASCs also improved restoration of skin architecture during wound healing.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views.
- Record: found
- Abstract: found
- Article: not found
Wound healing: an overview of acute, fibrotic and delayed healing.
- Record: found
- Abstract: found
- Article: not found
