- Record: found
- Abstract: found
- Article: found
Synthesis, Spectroscopic, X-ray Diffraction and DFT Studies of Novel Benzimidazole Fused-1,4-Oxazepines
Read this article at
Abstract
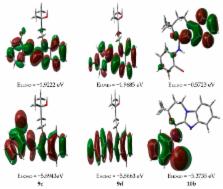
A series of benzimidazole-tethered oxazepine heterocyclic hybrids has been synthesized in good to excellent yields from an N-alkylated benzimidazole 2-carboxaldehyde, which in turn was accomplished from o-phenylenediamine in three good yielding steps. The calculated molecular structure of compounds 2-methyl-4-(2-((phenylimino)methyl)-1 H-benzo-[ d]imidazol-1-yl)-butan-2-ol 9 and 10 3,3-dimethyl- N-phenyl-1,2,3,5-tetrahydrobenzo-[4,5]imidazo[2,1- c][1,4]oxazepin-5-amine using the B3LYP/6–31 G(d, p) method were found to agree well with their X-ray structures. The charge distributions at the different atomic sites were computed using the natural bond orbital (NBO) method. The regions of electrophilic and nucleophilic reactivity were shown using a molecular electrostatic potential (MEP) map. In addition, the frontier molecular orbitals of these compounds were discussed at the same level of theory. Nonlinear optical (NLO) properties have also been investigated by computational hyperpolarizability studies, and it was found that Compound 9 is the best candidate for NLO applications.
Related collections
Most cited references37
- Record: found
- Abstract: not found
- Article: not found
A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons
- Record: found
- Abstract: found
- Article: not found
Origin of high second- and third-order nonlinear optical response in ammonio/borato diphenylpolyene zwitterions: the remarkable role of polarized aromatic groups.
- Record: found
- Abstract: found
- Article: not found