- Record: found
- Abstract: found
- Article: found
A 24-Week, Randomized, Treat-to-Target Trial Comparing Initiation of Insulin Glargine Once-Daily With Insulin Detemir Twice-Daily in Patients With Type 2 Diabetes Inadequately Controlled on Oral Glucose-Lowering Drugs
Read this article at
Abstract
OBJECTIVE
To determine whether glargine is noninferior to detemir regarding the percentage of patients reaching A1C <7% without symptomatic hypoglycemia ≤3.1 mmol/l.
RESEARCH DESIGN AND METHODS
In this 24-week trial, 973 insulin-naive type 2 diabetic patients on stable oral glucose-lowering drugs with A1C 7.0–10.5% were randomized to glargine once daily or detemir twice daily. Insulin doses were systematically titrated.
RESULTS
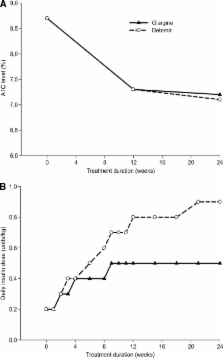
27.5 and 25.6% of patients reached the primary outcome with glargine and detemir, respectively, demonstrating the noninferiority of glargine. Improvements in A1C were −1.46 ± 1.09% for glargine and −1.54 ± 1.11% for detemir ( P = 0.149), with similar proportions of patients achieving A1C <7% ( P = 0.254) but more detemir-treated patients reaching A1C <6.5% ( P = 0.017). Hypoglycemia risk was similar. Weight gain was higher for glargine (difference: 0.77 kg, P < 0.001). Glargine doses were lower than detemir doses: 43.5 ± 29.0 vs. 76.5 ± 50.5 units/day ( P < 0.001).
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: not found
The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients.
- Record: found
- Abstract: found
- Article: not found
A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes
- Record: found
- Abstract: found
- Article: not found