- Record: found
- Abstract: found
- Article: found
Propagation of kinetic uncertainties through a canonical topology of the TLR4 signaling network in different regions of biochemical reaction space
Read this article at
Abstract
Background
Signal transduction networks represent the information processing systems that dictate which dynamical regimes of biochemical activity can be accessible to a cell under certain circumstances. One of the major concerns in molecular systems biology is centered on the elucidation of the robustness properties and information processing capabilities of signal transduction networks. Achieving this goal requires the establishment of causal relations between the design principle of biochemical reaction systems and their emergent dynamical behaviors.
Methods
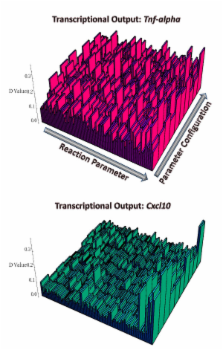
In this study, efforts were focused in the construction of a relatively well informed, deterministic, non-linear dynamic model, accounting for reaction mechanisms grounded on standard mass action and Hill saturation kinetics, of the canonical reaction topology underlying Toll-like receptor 4 (TLR4)-mediated signaling events. This signaling mechanism has been shown to be deployed in macrophages during a relatively short time window in response to lypopolysaccharyde (LPS) stimulation, which leads to a rapidly mounted innate immune response. An extensive computational exploration of the biochemical reaction space inhabited by this signal transduction network was performed via local and global perturbation strategies. Importantly, a broad spectrum of biologically plausible dynamical regimes accessible to the network in widely scattered regions of parameter space was reconstructed computationally. Additionally, experimentally reported transcriptional readouts of target pro-inflammatory genes, which are actively modulated by the network in response to LPS stimulation, were also simulated. This was done with the main goal of carrying out an unbiased statistical assessment of the intrinsic robustness properties of this canonical reaction topology.
Results
Our simulation results provide convincing numerical evidence supporting the idea that a canonical reaction mechanism of the TLR4 signaling network is capable of performing information processing in a robust manner, a functional property that is independent of the signaling task required to be executed. Nevertheless, it was found that the robust performance of the network is not solely determined by its design principle (topology), but this may be heavily dependent on the network's current position in biochemical reaction space. Ultimately, our results enabled us the identification of key rate limiting steps which most effectively control the performance of the system under diverse dynamical regimes.
Conclusions
Overall, our in silico study suggests that biologically relevant and non-intuitive aspects on the general behavior of a complex biomolecular network can be elucidated only when taking into account a wide spectrum of dynamical regimes attainable by the system. Most importantly, this strategy provides the means for a suitable assessment of the inherent variational constraints imposed by the structure of the system when systematically probing its parameter space.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
A methodology for performing global uncertainty and sensitivity analysis in systems biology.
- Record: found
- Abstract: found
- Article: not found
Robustness of cellular functions.
- Record: found
- Abstract: found
- Article: not found