- Record: found
- Abstract: found
- Article: found
Long noncoding RNA SNHG1 promotes cell proliferation through PI3K/AKT signaling pathway in pancreatic ductal adenocarcinoma
Read this article at
Abstract
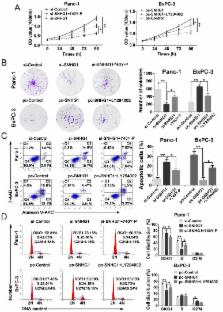
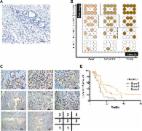
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most common causes of cancer-related death. Recently, long noncoding RNAs (lncRNAs) have emerged as significant regulators in numerous cancers, including PDAC. LncRNA small nucleolar RNA host gene 1 (SNHG1) has been reported in the development of several tumors, but the biological roles of it in PDAC remain to be illuminated. This study aims to investigate the function of lncRNA SNHG1, revealing its molecular mechanism and clinical significance in PDAC. Herein, we found that SNHG1 was highly expressed in PDAC tissues in comparison with adjacent noncancerous tissues, being closely related to tumor size and TNM stage. Functionally, silencing of SNHG1 could significantly inhibit cell proliferation, promote cell apoptosis, as well as alter cell cycle progression, whereas the contrary results could be presented in the overexpression of SNHG1. In addition, in vivo xenograft experiment also further confirmed the above results. Finally, an activator (740Y-P) and inhibitor (LY294002) of the PI3K/AKT signaling pathway were used in the western blot assays and the following rescue experiments, demonstrating that SNHG1 facilitates cell proliferation and tumorigenicity partly via the PI3K/AKT signaling pathway in PDAC. Hence, SNHG1 may be a prospective therapeutic target.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: found
The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer
- Record: found
- Abstract: found
- Article: found
Analysis of mortality rates for pancreatic cancer across the world
- Record: found
- Abstract: found
- Article: not found