- Record: found
- Abstract: found
- Article: found
Estimation of the risk for radiation-induced liver disease following photon- or proton-beam radiosurgery of liver metastases
Read this article at
Abstract
Background
Radiotherapy of liver metastases is commonly being performed with photon-beam based stereotactic body radiation therapy (SBRT). The high risk for radiation-induced liver disease (RILD) is a limiting factor in these treatments. The use of proton-beam based SBRT could potentially improve the sparing of the healthy part of the liver. The aim of this study was to use estimations of normal tissue complication probability (NTCP) to identify liver-metastases patients that could benefit from being treated with intensity-modulated proton therapy (IMPT), based on the reduction of the risk for RILD.
Methods
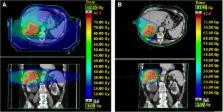
Ten liver metastases patients, previously treated with photon-beam based SBRT, were retrospectively planned with IMPT. A CTV-based robust optimisation (accounting for setup and range uncertainties), combined with a PTV-based conventional optimisation, was performed. A robustness criterion was defined for the CTV (V 95% > 98% for at least 10 of the 12 simulated scenarios). The NTCP was estimated for different endpoints using the Lyman-Kutcher-Burman model. The ΔNTCP ( NTCP IMPT − NTCP SBRT ) for RILD was registered for each patient. The patients for which the NTCP (RILD) < 5% were also identified. A generic relative biological effectiveness of 1.1 was assumed for the proton beams.
Results
For all patients, the objectives set for the PTV and the robustness criterion set for the CTV were fulfilled with the IMPT plans. An improved sparing of the healthy part of the liver, right kidney, lungs, spinal cord and the skin was achieved with the IMPT plans, compared to the SBRT plans. Mean liver doses larger than the threshold value of 32 Gy led to NTCP values for RILD exceeding 5% (7 patients with SBRT and 3 patients with the IMPT plans). ΔNTCP values (RILD) ranging between − 98% and − 17% (7 patients) and between 0 and 2% (3 patients), were calculated.
Conclusions
In this study, liver metastases patients that could benefit from being treated with IMPT, based on the NTCP reductions, were identified. The clinical implementation of such a model-based approach to select liver metastases patients to proton therapy needs to be made with caution while considering the uncertainties involved in the NTCP estimations.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Use of normal tissue complication probability models in the clinic.
- Record: found
- Abstract: found
- Article: not found
Radiotherapy combination opportunities leveraging immunity for the next oncology practice.
- Record: found
- Abstract: found
- Article: not found