- Record: found
- Abstract: found
- Article: found
Frequency and Associated Costs of Anaphylaxis- and Hypersensitivity-Related Adverse Events for Intravenous Iron Products in the USA: An Analysis Using the US Food and Drug Administration Adverse Event Reporting System
Read this article at
Abstract
Introduction and Objective
Intravenous iron preparations rapidly correct iron deficiency anemia, with the notable drug class effect of rare, yet potentially life-threatening, administration-related hypersensitivity or anaphylactic reactions. The objective of this comparative study was to assess adverse events associated with four intravenous iron preparations and estimated medical costs, in the US Food and Drug Administration Adverse Event Reporting System (FAERS) database.
Methods
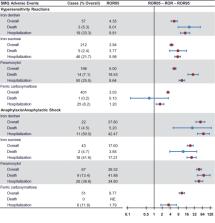
Cases of hypersensitivity reactions and anaphylaxis/anaphylactic shock associated with iron dextran, iron sucrose, ferumoxytol, and ferric carboxymaltose, spontaneously reported to FAERS (1 January, 2014 to 31 December, 2019), were extracted. The reporting odds ratio lower bound 90% confidence interval (ROR05) > 1 and cases ≥ 5 defined a likely signal for a drug–adverse event association. Adverse event-associated medical costs were estimated using Agency for Healthcare Research and Quality/Healthcare Cost and Utilization Project 2016 data.
Results
For hypersensitivity reactions, ferumoxytol and iron dextran had the highest ROR05 values (5.00 and 4.35, respectively) and greatest proportions of associated deaths (7.1% and 5.3%), followed by iron sucrose (ROR05 3.94, deaths 2.4%), and ferric carboxymaltose (ROR05 3.03, deaths 0.2%). For anaphylaxis/anaphylactic shock, ROR05 for cases/deaths were: 39.32/13.4%, ferumoxytol; 37.80/4.5%, iron dextran; 17.60/4.7%, iron sucrose; and 8.77/no deaths, ferric carboxymaltose. Downstream medical costs per adverse event were highest with iron dextran (US$8615) and ferumoxytol (US$8164), followed by iron sucrose (US$4212), and ferric carboxymaltose (US$1832).
Conclusions
Reporting rates of hypersensitivity and anaphylaxis with intravenous iron preparations were highest with ferumoxytol and lowest with ferric carboxymaltose in the US FAERS database. Adverse event-related medical costs were highest for iron dextran and ferumoxytol, and lowest for ferric carboxymaltose.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
A systematic analysis of global anemia burden from 1990 to 2010.
- Record: found
- Abstract: found
- Article: not found
A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions.
- Record: found
- Abstract: found
- Article: not found