- Record: found
- Abstract: found
- Article: found
Cytomegalovirus infection and disease reduce 10-year cardiac allograft vasculopathy-free survival in heart transplant recipients
Read this article at
Abstract
Background
Cytomegalovirus (CMV) is associated with an increased risk of cardiac allograft vasculopathy (CAV), the major limiting factor for long-term survival after heart transplantation (HTx). The purpose of this study was to evaluate the impact of CMV infection during long-term follow-up after HTx.
Methods
A retrospective, single-centre study analyzed 226 HTx recipients (mean age 45 ± 13 years, 78 % men) who underwent transplantation between January 1988 and December 2000. The incidence and risk factors for CMV infection during the first year after transplantation were studied. Risk factors for CAV were included in an analyses of CAV-free survival within 10 years post-transplant. The effect of CMV infection on the grade of CAV was analyzed.
Results
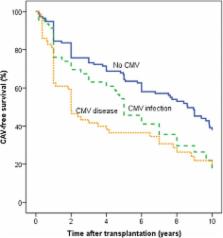
Survival to 10 years post-transplant was higher in patients with no CMV infection (69 %) compared with patients with CMV disease (55 %; p = 0.018) or asymptomatic CMV infection (54 %; p = 0.053). CAV-free survival time was higher in patients with no CMV infection (6.7 years; 95 % CI, 6.0–7.4) compared with CMV disease (4.2 years; CI, 3.2–5.2; p < 0.001) or asymptomatic CMV infection (5.4 years; CI, 4.3–6.4; p = 0.013). In univariate analysis, recipient age, donor age, coronary artery disease (CAD), asymptomatic CMV infection and CMV disease were significantly associated with CAV-free survival. In multivariate regression analysis, CMV disease, asymptomatic CMV infection, CAD and donor age remained independent predictors of CAV-free survival at 10 years post-transplant.
Conclusions
CAV-free survival was significantly reduced in patients with CMV disease and asymptomatic CMV infection compared to patients without CMV infection. These findings highlight the importance of close monitoring of CMV viral load and appropriate therapeutic strategies for preventing asymptomatic CMV infection.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection.
- Record: found
- Abstract: found
- Article: not found
Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival.
- Record: found
- Abstract: found
- Article: not found