- Record: found
- Abstract: found
- Article: not found
Light-Activated Olefin Metathesis: Catalyst Development, Synthesis, and Applications
Read this article at
Conspectus
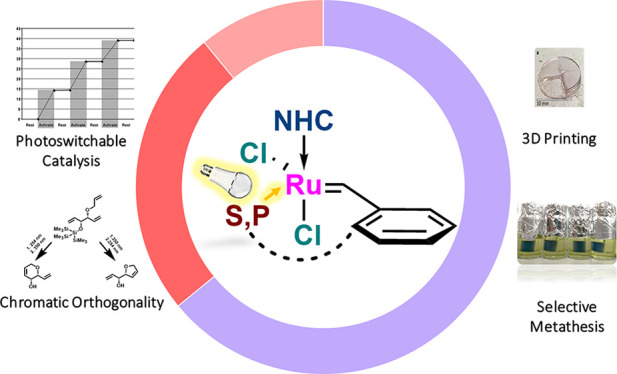
The most important means for tuning and improving a catalyst’s properties is the delicate exchange of the ligand shell around the central metal atom. Perhaps for no other organometallic-catalyzed reaction is this statement more valid than for ruthenium-based olefin metathesis. Indeed, even the simple exchange of an oxygen atom for a sulfur atom in a chelated ruthenium benzylidene about a decade ago resulted in the development of extremely stable, photoactive catalysts. This Account presents our perspective on the development of dormant olefin metathesis catalysts that can be activated by external stimuli and, more specifically, the use of light as an attractive inducing agent.
The insight gained from a deeper understanding of the properties of cis-dichlororuthenium benzylidenes opened the doorway for the systematic development of new and efficient light-activated olefin metathesis catalysts and catalytic chromatic-orthogonal synthetic schemes. Following this, ways to disrupt the ligand-to-metal bond to accelerate the isomerization process that produced the active precatalyst were actively pursued. Thus, we summarize herein the original thermal activation experiments and how they brought about the discoveries of photoactivation in the sulfur-chelated benzylidene family of catalysts. The specific wavelengths of light that were used to dissociate the sulfur–ruthenium bond allowed us to develop noncommutative catalytic chromatic-orthogonal processes and to combine other photochemical reactions with photoinduced olefin metathesis, including using external light-absorbing molecules as “sunscreens” to achieve novel selectivities. Alteration of the ligand sphere, including modifications of the N-heterocyclic carbene (NHC) ligand and the introduction of cyclic alkyl amino carbene (CAAC) ligands, produced more efficient light-induced activity and special chemical selectivity. The use of electron-rich sulfoxides and, more prominently, phosphites as the agents that induce latency widened the spectrum of light-induced olefin metathesis reactions even further by expanding the colors of light that may now be used to activate the catalysts, which can be used in applications such as stereolithography and 3D printing of tough metathesis-derived polymers.
Related collections
Most cited references59
- Record: found
- Abstract: not found
- Article: not found
Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference
- Record: found
- Abstract: found
- Article: not found
Mechanism and activity of ruthenium olefin metathesis catalysts.
- Record: found
- Abstract: found
- Article: not found
