- Record: found
- Abstract: found
- Article: found
The broad-spectrum chemokine inhibitor NR58-3.14.3 modulates macrophage-mediated inflammation in the diseased retina
Read this article at
Abstract
Background
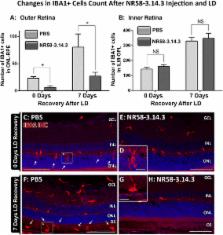
The activity of macrophages is implicated in the progression of retinal pathologies such as atrophic age-related macular degeneration (AMD), where they accumulate among the photoreceptor layer and subretinal space. This process is aided by the local expression of chemokines, which furnish these cells with directional cues that augment their migration to areas of retinal injury. While these qualities make chemokines a potential therapeutic target in curtailing damaging retinal inflammation, their wide variety and signalling redundancy pose challenges in broadly modulating their activity. Here, we examine the efficacy of the broad-spectrum chemokine inhibitor NR58-3.14.3—a suppressor of Ccl- and Cxcl- chemokine pathways—in suppressing macrophage activity and photoreceptor death, using a light-induced model of outer retinal atrophy and inflammation.
Methods
Photo-oxidative damage was induced in SD rats via exposure to 1000 lux of light for 24 h, after which animals were euthanized at 0- or 7-day post-exposure time points. Prior to damage, NR58-3.14.3 was injected intravitreally. Retinas were harvested and evaluated for the effect of NR58-3.14.3 on subretinal macrophage accumulation and cytokine expression profile, as well as photoreceptor degeneration.
Results
We report that intravitreal administration of NR58-3.14.3 reduces the accumulation of macrophages in the outer retina following exposure to light damage, at both 0- and 7-day post-exposure time points. Injection of NR58-3.14.3 also reduced the up-regulation of inflammatory markers including of Il6, Ccl3, and Ccl4 in infiltrating macrophages, which are promoters of their pathogenic activity in the retina. Finally, NR58-3.14.3-injected retinas displayed markedly reduced photoreceptor death following light damage, at both 0 and 7 days post-exposure.
Conclusions
Our findings indicate that NR58-3.14.3 is effective in inhibiting subretinal macrophage accumulation in light-induced retinal degeneration and illustrate the potential of broad-spectrum chemokine inhibitors as novel therapeutic agents in thwarting retinal inflammation. Although broad-spectrum chemokine inhibitors may not be appropriate for all retinal inflammatory conditions, our results suggest that they may be beneficial for retinal dystrophies in which chemokine expression and subretinal macrophage accumulation are implicated, such as advanced AMD.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited.
- Record: found
- Abstract: found
- Article: not found
Retinal microglia: just bystander or target for therapy?
- Record: found
- Abstract: found
- Article: not found