- Record: found
- Abstract: found
- Article: found
Ice VII from aqueous salt solutions: From a glass to a crystal with broken H-bonds
Read this article at
Abstract
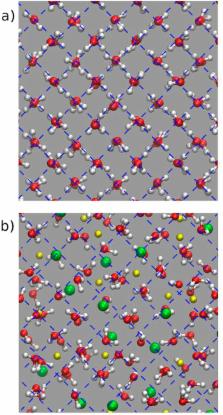
It has been known for decades that certain aqueous salt solutions of LiCl and LiBr readily form glasses when cooled to below ≈160 K. This fact has recently been exploited to produce a « salty » high-pressure ice form: When the glass is compressed at low temperatures to pressures higher than 4 GPa and subsequently warmed, it crystallizes into ice VII with the ionic species trapped inside the ice lattice. Here we report the extreme limit of salt incorporation into ice VII, using high pressure neutron diffraction and molecular dynamics simulations. We show that high-pressure crystallisation of aqueous solutions of LiCl∙RH 2O and LiBr∙RH 2O with R = 5.6 leads to solids with strongly expanded volume, a destruction of the hydrogen-bond network with an isotropic distribution of water-dipole moments, as well as a crystal-to-amorphous transition on decompression. This highly unusual behaviour constitutes an interesting pathway from a glass to a crystal where translational periodicity is restored but the rotational degrees of freedom remaining completely random.
Related collections
Most cited references10
- Record: found
- Abstract: not found
- Article: not found
Effect of ions on the structure of water: structure making and breaking.
- Record: found
- Abstract: found
- Article: not found