- Record: found
- Abstract: found
- Article: found
IL-33 Augments Virus-Specific Memory T Cell Inflation and Potentiates the Efficacy of an Attenuated Cytomegalovirus-Based Vaccine
Read this article at
Abstract
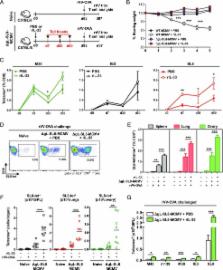
Candidate vaccines designed to generate T cell–based immunity are typically vectored by nonpersistent viruses, which largely fail to elicit durable effector memory T cell responses. This limitation can be overcome using recombinant strains of CMV. Proof-of-principle studies have demonstrated the potential benefits of this approach, most notably in the SIV model, but safety concerns require the development of nonreplicating alternatives with comparable immunogenicity. In this study, we show that IL-33 promotes the accumulation and recall kinetics of circulating and tissue-resident memory T cells in mice infected with murine CMV. Using a replication-deficient murine CMV vector, we further show that exogenous IL-33 boosts vaccine-induced memory T cell responses, which protect against subsequent heterologous viral challenge. These data suggest that IL-33 could serve as a useful adjuvant to improve the efficacy of vaccines based on attenuated derivatives of CMV.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention.
- Record: found
- Abstract: found
- Article: not found
Effector-memory T cell responses are associated with protection of rhesus monkeys from mucosal SIV challenge
- Record: found
- Abstract: found
- Article: not found