- Record: found
- Abstract: found
- Article: found
Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review
Read this article at
Abstract
Background
The availability of clinical and therapeutic data drawn from medical records and administrative databases has entailed new opportunities for clinical and epidemiologic research. However, these databases present inherent limitations which may render them prone to new biases. We aimed to conduct a structured review of biases specific to observational clinical studies based on secondary databases, and to propose strategies for the mitigation of those biases.
Methods
Scoping review of the scientific literature published during the period 2000–2018 through an automated search of MEDLINE, EMBASE and Web of Science, supplemented with manually cross-checking of reference lists. We included opinion essays, methodological reviews, analyses or simulation studies, as well as letters to the editor or retractions, the principal objective of which was to highlight the existence of some type of bias in pharmacoepidemiologic studies using secondary databases.
Results
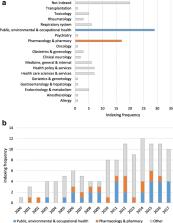
A total of 117 articles were included. An increasing trend in the number of publications concerning the potential limitations of secondary databases was observed over time and across medical research disciplines. Confounding was the most reported category of bias (63.2% of articles), followed by selection and measurement biases (47.0% and 46.2% respectively). Confounding by indication (32.5%), unmeasured/residual confounding (28.2%), outcome misclassification (28.2%) and “immortal time” bias (25.6%) were the subcategories most frequently mentioned.
Conclusions
Suboptimal use of secondary databases in pharmacoepidemiologic studies has introduced biases in the studies, which may have led to erroneous conclusions. Methods to mitigate biases are available and must be considered in the design, analysis and interpretation phases of studies using these data sources.
Related collections
Most cited references132
- Record: found
- Abstract: found
- Article: not found