- Record: found
- Abstract: found
- Article: found
Overexpression of Galectin 3 in Pancreatic β Cells Amplifies β-Cell Apoptosis and Islet Inflammation in Type-2 Diabetes in Mice
Read this article at
Abstract
Aims/Hypothesis: Galectin 3 appears to play a proinflammatory role in several inflammatory and autoimmune diseases. Also, there is evidence that galectin 3 plays a role in both type-1 and type-2 diabetes. During obesity, hematopoietic cell-derived galectin 3 induces insulin resistance. While the role of galectin 3 expressed in islet-invading immune cells in both type-1 and type-2 diabetes has been studied, the importance of the expression of this molecule on the target pancreatic β cells has not been defined.
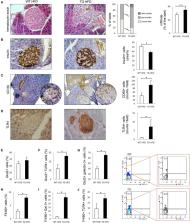
Methods: To clarify the role of galectin 3 expression in β cells during obesity-induced diabetogenesis, we developed transgenic mice selectively overexpressing galectin 3 in β cells and tested their susceptibility to obesity-induced type-2 diabetes. Obesity was induced with a 16-week high-fat diet regime. Pancreatic β cells were tested for susceptibility to apoptosis induced by non-esterified fatty acids and cytokines as well as parameters of oxidative stress.
Results: Our results demonstrated that overexpression of galectin 3 increases β-cell apoptosis in HFD conditions and increases the percentage of proinflammatory F4/80 + macrophages in islets that express galectin 3 and TLR4. In isolated islets, we have shown that galectin 3 overexpression increases cytokine and palmitate-triggered β-cell apoptosis and also increases NO 2−-induced oxidative stress of β cells. Also, in pancreatic lymph nodes, macrophages were shifted toward a proinflammatory TNF-α-producing phenotype.
Conclusions/Interpretation: By complementary in vivo and in vitro approaches, we have shown that galectin 3-overexpression facilitates β-cell damage, enhances cytokine and palmitate-triggered β-cell apoptosis, and increases NO 2−-induced oxidative stress in β cells. Further, the results suggest that increased expression of galectin 3 in the pancreatic β cells affects the metabolism of glucose and glycoregulation in mice on a high-fat diet, affecting both fasting glycemic values and glycemia after glucose loading.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Galectins as modulators of tumour progression.
- Record: found
- Abstract: found
- Article: not found
Galectin-3: an open-ended story.
- Record: found
- Abstract: found
- Article: not found