- Record: found
- Abstract: found
- Article: not found
Synthesis of E- and Z-trisubstituted alkenes by catalytic cross-metathesis
Read this article at
Abstract
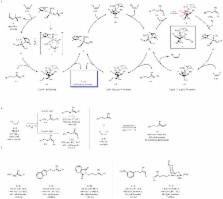
Catalytic cross-metathesis is a central transformation in chemistry, and yet, corresponding methods for stereoselectively generating acyclic trisubstituted alkenes in either isomeric form do not exist. The key problems are lack of chemoselectivity, namely, the preponderance of side reactions involving only the less hindered starting alkene, ensuing nonproductive processes of homo-metathesis byproducts, and formation of short-lived methylidene complexes. In contrast, in catalytic cross-coupling, another widely used process, substrates are more distinct and homocoupling is less of a problem. Here, we show that through cross-metathesis reactions involving E- or a Z-trisubstituted alkenes, easily prepared from commercially available starting materials by cross-coupling processes, many otherwise desirable and difficult-to-access linear E- or Z-trisubstituted alkenes can be synthesized efficiently and in exceptional stereoisomeric purity (up to >98% E or 95% Z). Utility is highlighted through concise stereoselective syntheses of biologically active compounds such as indiacen B (anti-fungal) and coibacin D (anti-inflammatory).
Related collections
Most cited references46
- Record: found
- Abstract: not found
- Article: not found
The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects
- Record: found
- Abstract: found
- Article: not found
The remarkable metal-catalysed olefin metathesis reaction.
- Record: found
- Abstract: found
- Article: not found
