- Record: found
- Abstract: found
- Article: found
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the CGRP Binding Monoclonal Antibody LY2951742 (Galcanezumab) in Healthy Volunteers
Read this article at
Abstract
Background: Calcitonin gene-related peptide (CGRP) is pivotal in the pathophysiology of migraine headaches and represents a promising target for migraine treatment. The humanized monoclonal antibody galcanezumab (LY2951742) binds to CGRP and may be effective in migraine prophylaxis.
Objectives: The primary objective was to evaluate the safety and tolerability of single and multiple doses of galcanezumab in humans. Secondary objectives included assessing the pharmacokinetics and evaluating target engagement.
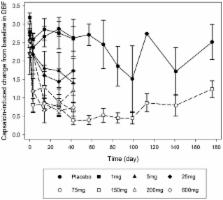
Methods: A double-blind, randomized, placebo-controlled study (NCT 01337596) with single escalating and multiple subcutaneous (SC) doses of galcanezumab was performed in healthy male volunteers. Single doses of 1, 5, 25, 75, 200, and 600 mg of galcanezumab ( n = 7/dose) or placebo ( n = 2/dose) were injected SC in six consecutive cohorts of nine subjects each. One cohort of nine subjects received multiple (4) 150 mg doses of galcanezumab or placebo every other week. Target engagement was evaluated by measuring inhibition of capsaicin-induced increase in dermal blood flow (DBF).
Findings: Sixty-three subjects were randomized and included in the safety analyses. Galcanezumab was well tolerated in single doses (1–600 mg SC) and consecutive doses (150 mg SC). There was no dose-dependent difference in type or frequency of treatment-emergent adverse events, and no clinically meaningful difference when compared with placebo. Pharmacokinetics were linear. Galcanezumab induced a robust, dose-dependent, and durable inhibition of capsaicin-induced increase in DBF, supporting the continued clinical development of galcanezumab for prophylaxis in migraine patients.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
CGRP may play a causative role in migraine.
- Record: found
- Abstract: found
- Article: not found
CGRP and its receptors provide new insights into migraine pathophysiology.
- Record: found
- Abstract: found
- Article: not found