- Record: found
- Abstract: found
- Article: found
Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in an STI population: performances of the Presto CT-NG assay, the Lightmix Kit 480 HT CT/NG and the COBAS Amplicor with urine specimens and urethral/cervicovaginal samples
Read this article at
Abstract
Objectives
This study assessed the performances of the Presto CT-NG assay, the Lightmix Kit 480 HT CT/NG and the COBAS Amplicor for Chlamydia trachomatis and Neisseria gonorrhoeae detection.
Setting
Izore, Centre for Diagnosing Infectious Diseases in Friesland, the Netherlands, tested samples sent from regional sexually transmitted infection (STI) outpatient clinics and regional hospitals from the province Friesland, the Netherlands.
Participants
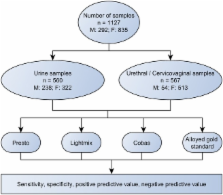
Samples were collected from 292 men and 835 women. These samples included 560 urine samples and 567 urethral/cervicovaginal samples.
Primary and secondary outcome measures
The primary outcome measure is C trachomatis infection. No secondary outcome measures are available.
Results
The sensitivity, specificity, positive predicative value (PPV) and negative predictive value (NPV) for C trachomatis detection in urine samples using the Presto CT-NG assay were 100%, 99.8%, 98.1% and 100%, respectively; for the Lightmix Kit 480 HT CT/NG: 94.2%, 99.8%, 96.1% and 99.4%, respectively; for the COBAS Amplicor: 92.3%, 99.6%, 96% and 99.2%, respectively. The sensitivity, specificity, PPV and NPV for C trachomatis detection in urethral/cervicovaginal swabs using the Presto CT-NG assay and the COBAS Amplicor were 100%, 99.8%, 97.7% and 100%, respectively; for the Lightmix Kit 480 HT CT/NG: 100%, 99.6%, 97.7% and 100%, respectively. Calculations for N gonorrhoeae could not be made due to a low prevalence.
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Measurement error correction for logistic regression models with an "alloyed gold standard".
- Record: found
- Abstract: found
- Article: not found
Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea.
- Record: found
- Abstract: found
- Article: not found