- Record: found
- Abstract: found
- Article: found
Towards Personalized Cardiology: Multi-Scale Modeling of the Failing Heart
Read this article at
Abstract
Background
Despite modern pharmacotherapy and advanced implantable cardiac devices, overall prognosis and quality of life of HF patients remain poor. This is in part due to insufficient patient stratification and lack of individualized therapy planning, resulting in less effective treatments and a significant number of non-responders.
Methods and Results
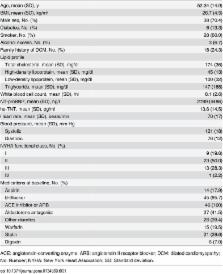
State-of-the-art clinical phenotyping was acquired, including magnetic resonance imaging (MRI) and biomarker assessment. An individualized, multi-scale model of heart function covering cardiac anatomy, electrophysiology, biomechanics and hemodynamics was estimated using a robust framework. The model was computed on n=46 HF patients, showing for the first time that advanced multi-scale models can be fitted consistently on large cohorts. Novel multi-scale parameters derived from the model of all cases were analyzed and compared against clinical parameters, cardiac imaging, lab tests and survival scores to evaluate the explicative power of the model and its potential for better patient stratification. Model validation was pursued by comparing clinical parameters that were not used in the fitting process against model parameters.
Conclusion
This paper illustrates how advanced multi-scale models can complement cardiovascular imaging and how they could be applied in patient care. Based on obtained results, it becomes conceivable that, after thorough validation, such heart failure models could be applied for patient management and therapy planning in the future, as we illustrate in one patient of our cohort who received CRT-D implantation.
Related collections
Most cited references28
- Record: found
- Abstract: not found
- Article: not found
ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC.
- Record: found
- Abstract: found
- Article: not found
Whole-heart modeling: applications to cardiac electrophysiology and electromechanics.
- Record: found
- Abstract: found
- Article: not found