- Record: found
- Abstract: found
- Article: found
Multi-omics analysis reveals GABAergic dysfunction after traumatic brainstem injury in rats
Read this article at
Abstract
Background
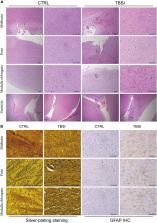
Traumatic brainstem injury (TBSI) is one of the forms of brain injury and has a very high mortality rate. Understanding the molecular mechanism of injury can provide additional information for clinical treatment.
Materials and methods
In this study, we detected transcriptome, proteomics, and metabolome expression changes in the brainstem of TBSI rats, and comprehensively analyzed the underlying mechanisms of TBSI.
Results
After TBSI, there was significant diffuse axonal injury (DAI) in the brainstem of rats. A total of 579 genes, 70 proteins, and 183 metabolites showed significant changes in brainstem tissue. Through molecular function and pathway analysis, the differentially expressed genes, proteins, and metabolites of TBSI were mainly attributed to neural signal regulation, inflammation, neuroprotection, and immune system. In addition, a comprehensive analysis of transcripts, proteins, and metabolites showed that the genes, proteins, and metabolic pathways regulated in the brainstem after TBSI were involved in neuroactive ligand-receptor interaction. A variety of GCPR-regulated pathways were affected, especially GAGA’s corresponding receptors GABA A, GABA B, GABA C, and transporter GAT that were inhibited to varying degrees.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain.
- Record: found
- Abstract: found
- Article: not found