- Record: found
- Abstract: found
- Article: found
Endometrium and endometriosis tissue mitochondrial energy metabolism in a nonhuman primate model
Read this article at
Abstract
Background
Endometriosis is the growth of uterine lining (endometrium) outside of the uterus. In other chronic inflammatory diseases, mitochondrial dysfunction is suspected of playing a role in disease pathogenesis. However, little is known about endometriosis mitochondrial function or its effects on tissue metabolism. The objectives of this study were to analyze mitochondrial function in nonhuman primate (NHP) endometrium and endometriosis tissue and to identify the metabolic features of these tissues that may contribute to disease.
Methods
Mitochondrial function in endometriosis tissue and endometrium was measured using mitochondrial respirometry analysis to determine if changes in oxidative phosphorylation exist in endometrium and endometriosis tissue compared to control endometrium from clinically healthy NHPs. Targeted metabolomics and multidimensional statistical analysis were applied to quantify key metabolites in energy and amino acid biosynthesis pathways.
Results
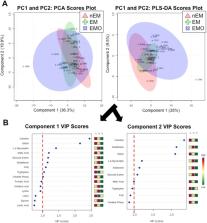
Mitochondrial respirometry assays showed endometrium from NHPs with endometriosis had reduced complex II-mediated oxygen consumption rates (OCR) across all energy states (basal, p = 0.01; state 3, p = 0.02; state 3u, p = 0.04; state 4o, p = 0.008) and endometriosis tissue had reduced state 3, complex I-mediated OCR ( p = 0.02) and respiratory control rates ( p = 0.01) compared to normal endometrium. Targeted metabolomics performed on tissue revealed carnitine ( p = 0.001), creatine phosphate ( p = 0.01), NADH ( p = 0.0001), FAD ( p = 0.001), tryptophan ( p = 0.0009), and malic acid ( p = 0.005) were decreased in endometriosis tissue compared to normal endometrium samples. FAD ( p = 0.004), tryptophan ( p = 0.0004) and malic acid ( p = 0.03) were significantly decreased in endometrium from NHPs with endometriosis compared to normal endometrium. Significant metabolites identified in endometriosis and endometrium samples from animals with endometriosis were part of amino acid biosynthesis or energy metabolism pathways.
Conclusions
Here, endometrial mitochondrial energy production and metabolism were decreased in endometrium and endometriosis tissue. Decreased mitochondrial energy production may be due to oxidative stress-induced damage to mitochondrial DNA or membranes, a shift in cell metabolism, or decreased energy substrate; however, the exact cause remains unknown. Additional research is needed to determine the implications of reduced mitochondrial energy production and metabolism on endometriosis and endometrium.
Related collections
Most cited references28

- Record: found
- Abstract: found
- Article: found
MetaboAnalyst: a web server for metabolomic data analysis and interpretation
- Record: found
- Abstract: found
- Article: not found
Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging.

- Record: found
- Abstract: found
- Article: found