- Record: found
- Abstract: found
- Article: found
The stability and activity of human neuroserpin are modulated by a salt bridge that stabilises the reactive centre loop
Read this article at
Abstract
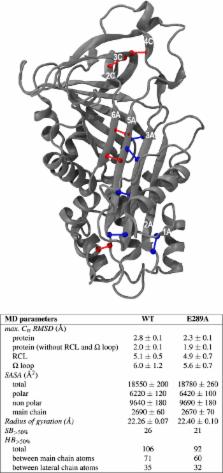
Neuroserpin (NS) is an inhibitory protein belonging to the serpin family and involved in several pathologies, including the dementia Familial Encephalopathy with Neuroserpin Inclusion Bodies (FENIB), a genetic neurodegenerative disease caused by accumulation of NS polymers. Our Molecular Dynamics simulations revealed the formation of a persistent salt bridge between Glu289 on strand s2C and Arg362 on the Reactive Centre Loop (RCL), a region important for the inhibitory activity of NS. Here, we validated this structural feature by simulating the Glu289Ala mutant, where the salt bridge is not present. Further, MD predictions were tested in vitro by purifying recombinant Glu289Ala NS from E. coli. The thermal and chemical stability along with the polymerisation propensity of both Wild Type and Glu289Ala NS were characterised by circular dichroism, emission spectroscopy and non-denaturant gel electrophoresis, respectively. The activity of both variants against the main target protease, tissue-type plasminogen activator (tPA), was assessed by SDS-PAGE and chromogenic kinetic assay. Our results showed that deletion of the salt bridge leads to a moderate but clear reduction of the overall protein stability and activity.
Related collections
Most cited references21
- Record: found
- Abstract: not found
- Article: not found
The interpretation of protein structures: estimation of static accessibility.
- Record: found
- Abstract: found
- Article: not found
Wordom: a program for efficient analysis of molecular dynamics simulations.
- Record: found
- Abstract: found
- Article: not found