- Record: found
- Abstract: found
- Article: found
Structural and functional characterization of two alpha-synuclein strains
Read this article at
Abstract
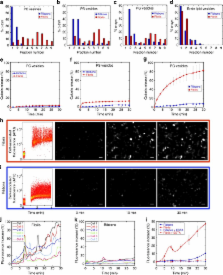
α-synuclein aggregation is implicated in a variety of diseases including Parkinson’s disease, dementia with Lewy bodies, pure autonomic failure and multiple system atrophy. The association of protein aggregates made of a single protein with a variety of clinical phenotypes has been explained for prion diseases by the existence of different strains that propagate through the infection pathway. Here we structurally and functionally characterize two polymorphs of α-synuclein. We present evidence that the two forms indeed fulfil the molecular criteria to be identified as two strains of α-synuclein. Specifically, we show that the two strains have different structures, levels of toxicity, and in vitro and in vivo seeding and propagation properties. Such strain differences may account for differences in disease progression in different individuals/cell types and/or types of synucleinopathies.
Abstract
 α-synuclein is implicated in neurodegenerative diseases. Bousset
et al. generate two α-synuclein polymorphs and find differences in aggregation, function
and toxicity, suggesting that these altered properties may be the cause for differences
in disease progression.
α-synuclein is implicated in neurodegenerative diseases. Bousset
et al. generate two α-synuclein polymorphs and find differences in aggregation, function
and toxicity, suggesting that these altered properties may be the cause for differences
in disease progression.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Stages in the development of Parkinson's disease-related pathology.
- Record: found
- Abstract: found
- Article: not found
alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies.
- Record: found
- Abstract: found
- Article: not found