- Record: found
- Abstract: found
- Article: found
International Comparisons of Harmonized Laboratory Value Trajectories to Predict Severe COVID-19: Leveraging the 4CE Collaborative Across 342 Hospitals and 6 Countries: A Retrospective Cohort Study
Read this article at
Abstract
Objectives:
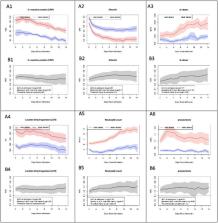
To perform an international comparison of the trajectory of laboratory values among hospitalized patients with COVID-19 who develop severe disease and identify optimal timing of laboratory value collection to predict severity across hospitals and regions.
Setting:
The Consortium for Clinical Characterization of COVID-19 by EHR (4CE), an international multi-site data-sharing collaborative of 342 hospitals in the US and in Europe.
Participants:
Patients hospitalized with COVID-19, admitted before or after PCR-confirmed result for SARS-CoV-2.
Primary and secondary outcome measures:
Patients were categorized as “ever-severe” or “never-severe” using the validated 4CE severity criteria. Eighteen laboratory tests associated with poor COVID-19-related outcomes were evaluated for predictive accuracy by area under the curve (AUC), compared between the severity categories. Subgroup analysis was performed to validate a subset of laboratory values as predictive of severity against a published algorithm. A subset of laboratory values (CRP, albumin, LDH, neutrophil count, D-dimer, and procalcitonin) was compared between North American and European sites for severity prediction.
Results:
Of 36,447 patients with COVID-19, 19,953 (43.7%) were categorized as ever-severe. Most patients (78.7%) were 50 years of age or older and male (60.5%). Longitudinal trajectories of CRP, albumin, LDH, neutrophil count, D-dimer, and procalcitonin showed association with disease severity. Significant differences of laboratory values at admission were found between the two groups. With the exception of D-dimer, predictive discrimination of laboratory values did not improve after admission. Sub-group analysis using age, D-dimer, CRP, and lymphocyte count as predictive of severity at admission showed similar discrimination to a published algorithm (AUC=0.88 and 0.91, respectively). Both models deteriorated in predictive accuracy as the disease progressed. On average, no difference in severity prediction was found between North American and European sites.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Clinical Characteristics of Coronavirus Disease 2019 in China
- Record: found
- Abstract: not found
- Article: not found
