- Record: found
- Abstract: found
- Article: found
Heritability of individualized cortical network topography
Read this article at
Significance
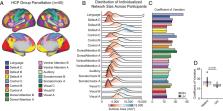
The widespread use of population-average cortical parcellations has provided important insights into broad properties of human brain organization. However, the size, location, and spatial arrangement of regions comprising functional brain networks can vary substantially across individuals. Here, we demonstrate considerable heritability in both the size and spatial organization of individual-specific network topography across cortex. Genetic factors had a regionally variable influence on brain organization, such that heritability in network size, but not topography, was greater in unimodal relative to heteromodal cortices. These data suggest individual-specific network parcellations may provide an avenue to understand the genetic basis of variation in human cognition and behavior.
Abstract
Human cortex is patterned by a complex and interdigitated web of large-scale functional networks. Recent methodological breakthroughs reveal variation in the size, shape, and spatial topography of cortical networks across individuals. While spatial network organization emerges across development, is stable over time, and is predictive of behavior, it is not yet clear to what extent genetic factors underlie interindividual differences in network topography. Here, leveraging a nonlinear multidimensional estimation of heritability, we provide evidence that individual variability in the size and topographic organization of cortical networks are under genetic control. Using twin and family data from the Human Connectome Project ( n = 1,023), we find increased variability and reduced heritability in the size of heteromodal association networks ( h 2 : M = 0.34, SD = 0.070), relative to unimodal sensory/motor cortex ( h 2 : M = 0.40, SD = 0.097). We then demonstrate that the spatial layout of cortical networks is influenced by genetics, using our multidimensional estimation of heritability ( h 2 -multi; M = 0.14, SD = 0.015). However, topographic heritability did not differ between heteromodal and unimodal networks. Genetic factors had a regionally variable influence on brain organization, such that the heritability of network topography was greatest in prefrontal, precuneus, and posterior parietal cortex. Taken together, these data are consistent with relaxed genetic control of association cortices relative to primary sensory/motor regions and have implications for understanding population-level variability in brain functioning, guiding both individualized prediction and the interpretation of analyses that integrate genetics and neuroimaging.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
The organization of the human cerebral cortex estimated by intrinsic functional connectivity.
- Record: found
- Abstract: found
- Article: not found
The minimal preprocessing pipelines for the Human Connectome Project.
- Record: found
- Abstract: found
- Article: not found