- Record: found
- Abstract: found
- Article: found
Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer
Read this article at
Abstract
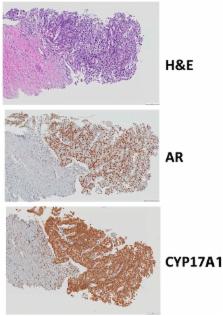
Cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) is a validated treatment target for the treatment of metastatic castration-resistant prostate cancer (CRPC). Abiraterone acetate (AA) inhibits both 17α-hydroxylase (hydroxylase) and 17,20-lyase (lyase) reactions catalyzed by CYP17A1 and thus depletes androgen biosynthesis. However, coadministration of prednisone is required to suppress the mineralocorticoid excess and cortisol depletion that result from hydroxylase inhibition. VT-464, a nonsteroidal small molecule, selectively inhibits CYP17A1 lyase and therefore does not require prednisone supplementation. Administration of VT-464 in a metastatic CRPC patient presenting with high tumoral expression of both androgen receptor (AR) and CYP17A1, showed significant reduction in the level of both dehydroepiandrosterone (DHEA) and serum PSA. Treatment of a CRPC patient-derived xenograft, MDA-PCa-133 expressing H874Y AR mutant with VT-464, reduced the increase in tumor volume in castrate male mice more than twice as much as the vehicle (P < 0.05). Mass spectrometry analysis of post-treatment xenograft tumor tissues showed that VT-464 significantly decreased intratumoral androgens but not cortisol. VT-464 also reduced AR signaling more effectively than abiraterone in cultured PCa cells expressing T877A AR mutant. Collectively, this study suggests that VT-464 therapy can effectively treat CRPC and be used in precision medicine based on androgen receptor mutation status.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1.
- Record: found
- Abstract: found
- Article: not found
Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants.
- Record: found
- Abstract: found
- Article: not found