- Record: found
- Abstract: found
- Article: found
Hepatic zonation of carbon and nitrogen fluxes derived from glutamine and ammonia transformations
Read this article at
Abstract
Background
Glutaminase predominates in periportal hepatocytes and it has been proposed that it determines the glutamine-derived nitrogen flow through the urea cycle. Glutamine-derived urea production should, thus, be considerably faster in periportal hepatocytes. This postulate, based on indirect observations, has not yet been unequivocally demonstrated, making a direct investigation of ureogenesis from glutamine highly desirable.
Methods
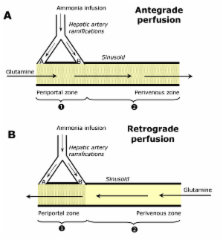
Zonation of glutamine metabolism was investigated in the bivascularly perfused rat liver with [U- 14C]glutamine infusion (0.6 mM) into the portal vein (antegrade perfusion) or into the hepatic vein (retrograde perfusion).
Results
Ammonia infusion into the hepatic artery in retrograde and antegrade perfusion allowed to promote glutamine metabolism in the periportal region and in the whole liver parenchyma, respectively. The results revealed that the space-normalized glutamine uptake, indicated by 14CO 2 production, gluconeogenesis, lactate production and the associated oxygen uptake, predominates in the periportal region. Periportal predominance was especially pronounced for gluconeogenesis. Ureogenesis, however, tended to be uniformly distributed over the whole liver parenchyma at low ammonia concentrations (up to 1.0 mM); periportal predominance was found only at ammonia concentrations above 1 mM. The proportions between the carbon and nitrogen fluxes in periportal cells are not the same along the liver acinus.
Conclusions
In conclusion, the results of the present work indicate that the glutaminase activity in periportal hepatocytes is not the rate-controlling step of the glutamine-derived nitrogen flow through the urea cycle. The findings corroborate recent work indicating that ureogenesis is also an important ammonia-detoxifying mechanism in cells situated downstream to the periportal region.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Differential gene expression in periportal and perivenous mouse hepatocytes.
- Record: found
- Abstract: found
- Article: not found
Role of glutamine in human carbohydrate metabolism in kidney and other tissues.
- Record: found
- Abstract: not found
- Article: not found