- Record: found
- Abstract: found
- Article: found
Relapsed/refractory acquired thrombotic thrombocytopenic purpura in a patient with Sjögren syndrome : Case report and review of the literature
Read this article at
Abstract
Rationale:
Thrombotic thrombocytopenic purpura (TTP) is a rare, fatal disorder which could be caused by autoimmune diseases. However, TTP secondary to Sjögren syndrome (SS) is extremely rare.
Patient concerns:
A 47-year- old woman with an 8-year history of SS was admitted due to skin ecchymosis and bleeding gums. Then she gradually developed fever and headache.
Diagnoses:
Laboratory investigations suggested anemia, thrombocytopenia, increased lactic dehydrogenase, and a disintegrin-like metalloproteinase with thrombospondin motif type 1 member 13 (ADAMTS13) activity deficiency with high inhibitor titers. Acquired TTP was thus diagnosed.
Interventions:
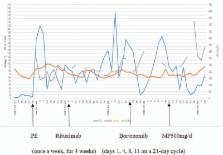
Plasma exchange (PE) was the first choice for treatment, while glucocorticoid, cyclosporine A (CSA), rituximab, and intravenous immunoglobulin (IVIG) were used simultaneously. Bortezomib, a selective proteasome inhibitor and thereby inducing apoptosis in both B-cells and plasma cells, was added.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients.
- Record: found
- Abstract: found
- Article: not found
Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection.
- Record: found
- Abstract: not found
- Article: not found