- Record: found
- Abstract: found
- Article: found
Variations in microbiota composition of laboratory mice influence Citrobacter rodentium infection via variable short-chain fatty acid production
Read this article at
Abstract
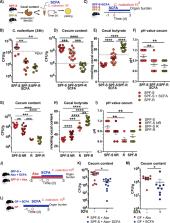
The composition of the intestinal microbiota influences the outcome of enteric infections in human and mice. However, the role of specific members and their metabolites contributing to disease severity is largely unknown. Using isogenic mouse lines harboring distinct microbiota communities, we observed highly variable disease kinetics of enteric Citrobacter rodentium colonization after infection. Transfer of communities from susceptible and resistant mice into germ-free mice verified that the varying susceptibilities are determined by microbiota composition. The strongest differences in colonization were observed in the cecum and could be maintained in vitro by coculturing cecal bacteria with C. rodentium. Cohousing of animals as well as the transfer of cultivable bacteria from resistant to susceptible mice led to variable outcomes in the recipient mice. Microbiome analysis revealed that a higher abundance of butyrate-producing bacteria was associated with the resistant phenotype. Quantification of short-chain fatty acid (SCFA) levels before and after infection revealed increased concentrations of acetate, butyrate and propionate in mice with delayed colonization. Addition of physiological concentrations of butyrate, but not of acetate and/or propionate strongly impaired growth of C. rodentium in vitro. In vivo supplementation of susceptible, antibiotic-treated and germ-free mice with butyrate led to the same level of protection, notably only when cecal butyrate concentration reached a concentration higher than 50 nmol/mg indicating a critical threshold for protection. In the recent years, commensal-derived primary and secondary bacterial metabolites emerged as potent modulators of hosts susceptibility to infection. Our results provide evidence that variations in SCFA production in mice fed fibre-rich chow-based diets modulate susceptibility to colonization with Enterobacteriaceae not only in antibiotic-disturbed ecosystems but even in undisturbed microbial communities. These findings emphasise the need for microbiota normalization across laboratory mouse lines for infection experiments with the model-pathogen C. rodentium independent of investigations of diet and antibiotic usage.
Author summary
The distinct composition of the gut microbiota in each individual results in variable metabolic activity and output of these communities, which influences the host, including resistance to enteric pathogens. Lack of reproducibility in biomedical research is nowadays frequently attributed to the microbiota, but little is known about which specific members and metabolites contribute to disease severity. Here, we use genetically identical mouse lines with variable microbiota compositions on a standardized diet and observed highly variable colonization with the enteric pathogen Citrobacter rodentium without antibiotics intervention. We found the same differences in formerly germ-free animals harbouring the respective donors microbiota and also in vitro by coculturing cecal bacteria from resistant and susceptible animals with C. rodentium showing that the phenotype is fully dependent on differences in the microbiota. We analysed the microbiome composition and found a higher abundance of butyrate-producing bacteria as well as increased levels of butyrate in resistant mice. By supplementation of susceptible and germ-free animals with butyrate, we could significantly lower the levels of colonization highlighting that commensal-derived primary and secondary bacterial metabolites are highly variable between laboratory animals from different vendors and are potent modulators of hosts susceptibility to infection with C. rodentium.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample.
- Record: found
- Abstract: found
- Article: not found
Microbiota-mediated colonization resistance against intestinal pathogens.
- Record: found
- Abstract: found
- Article: not found