- Record: found
- Abstract: found
- Article: found
Modes of tetra(4-pyridyl)porphyrinatomanganese(III) ion intercalation inside natural clays
Read this article at
Abstract
Background
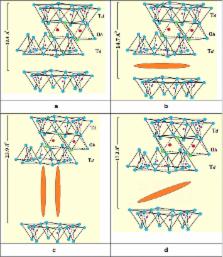
Metalloporphyrin ions, with planar shape, have been known to intercalate horizontally and diagonally between montmorillonite layers. Perpendicular intercalation inside montmorillonite has not been reported earlier. This work aims at achieving perpendicular intercalation inside montmorillonite in natural clays. Possible intercalation inside other forms of natural clay will also be investigated.
Methods
Natural clays were purified and characterized. The naked clay powder was then refluxed with tetra(4-pyridyl)porphyrinatomanganese(III) ion (MnTPyP +) solution in methanol with continuous stirring for different times. Electronic absorption spectra, atomic absorption spectra, Fourier Transform infrared spectra, scanning electron microscopy and X-ray diffraction were all used in clay characterization and in intercalation study.
Results
The natural clay involved different phases, namely montmorillonite, biotite, kaolinite, illite and traces of quartz. Montmorillonite clay allowed horizontal, diagonal and perpendicular intercalation of the metalloporphyrin ions. Biotite allowed only horizontal intercalation. The mode of intercalation was deduced by monitoring the clay inter-planar distance value change. Intercalation occurred inside both micro- and nano-size clay powders to different extents. The nano-powder (average size ~50 nm) showed uptake values up to 3.8 mg MnTPyP/g solid, whereas the micro-size powder (average size ~316 nm) exhibited lower uptake (2.4 mg MnTPyP/g solid). Non-expandable clay phases did not allow any intercalation. The intercalated MnTPyP + ions showed promising future supported catalyst applications.
Conclusions
Depending on their phase, natural clays hosted metalloporphyrin ions. Montmorillonite can allow all three possible intercalation geometries, horizontal, diagonal and for the first time perpendicular. Biotite allows horizontal intercalation only. Non-expandable clays allow no intercalation.
Related collections
Most cited references42
- Record: found
- Abstract: not found
- Article: not found
Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view
- Record: found
- Abstract: not found
- Article: not found
