- Record: found
- Abstract: found
- Article: found
NF-κB Signaling Regulates Expression of Epstein-Barr Virus BART MicroRNAs and Long Noncoding RNAs in Nasopharyngeal Carcinoma
Read this article at
ABSTRACT
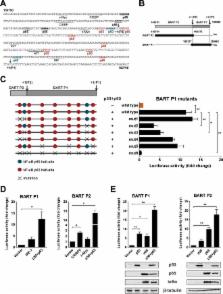
Epstein-Barr virus (EBV) expresses few viral proteins in nasopharyngeal carcinoma (NPC) but high levels of BamHI-A rightward transcripts (BARTs), which include long noncoding RNAs (lncRNAs) and BART microRNAs (miRNAs). It is hypothesized that the mechanism for regulation of BARTs may relate to EBV pathogenesis in NPC. We showed that nuclear factor-κB (NF-κB) activates the BART promoters and modulates the expression of BARTs in EBV-infected NPC cells but that introduction of mutations into the putative NF-κB binding sites abolished activation of BART promoters by NF-κB. Binding of p50 subunits to NF-κB sites in the BART promoters was confirmed in electrophoretic mobility shift assays (EMSA) and further demonstrated in vivo using chromatin immunoprecipitation (ChIP) analysis. Expression of BART miRNAs and lncRNAs correlated with NF-κB activity in EBV-infected epithelial cells, while treatment of EBV-harboring NPC C666-1 cells with aspirin (acetylsalicylic acid [ASA]) and the IκB kinase inhibitor PS-1145 inhibited NF-κB activity, resulting in downregulation of BART expression. Expression of EBV LMP1 activates BART promoters, whereas an LMP1 mutant which cannot induce NF-κB activation does not activate BART promoters, further supporting the idea that expression of BARTs is regulated by NF-κB signaling. Expression of LMP1 is tightly regulated in NPC cells, and this study confirmed that miR-BART5-5p downregulates LMP1 expression, suggesting a feedback loop between BART miRNA and LMP1-mediated NF-κB activation in the NPC setting. These findings provide new insights into the mechanism underlying the deregulation of BARTs in NPC and identify a regulatory loop through which BARTs support EBV latency in NPC.
IMPORTANCE Nasopharyngeal carcinoma (NPC) cells are ubiquitously infected with Epstein-Barr virus (EBV). Notably, EBV expresses very few viral proteins in NPC cells, presumably to avoid triggering an immune response, but high levels of EBV BART miRNAs and lncRNAs which exhibit complex functions associated with EBV pathogenesis. The mechanism for regulation of BARTs is critical for understanding NPC oncogenesis. This study provides multiple lines of evidence to show that expression of BARTs is subject to regulation by NF-κB signaling. EBV LMP1 is a potent activator of NF-κB signaling, and we demonstrate that LMP1 can upregulate expression of BARTs through NF-κB signaling and that BART miRNAs are also able to downregulate LMP1 expression. It appears that aberrant NF-κB signaling and expression of BARTs form an autoregulatory loop for maintaining EBV latency in NPC cells. Further exploration of how targeting NF-κB signaling interrupts EBV latency in NPC cells may reveal new options for NPC treatment.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
NF-kappa B as a therapeutic target in multiple myeloma.
- Record: found
- Abstract: found
- Article: not found
Epstein-Barr virus-associated gastric adenocarcinoma.
- Record: found
- Abstract: found
- Article: not found