- Record: found
- Abstract: found
- Article: found
VITAL phase 2 study: Upfront 5‐fluorouracil, mitomycin‐C, panitumumab and radiotherapy treatment in nonmetastatic squamous cell carcinomas of the anal canal (GEMCAD 09‐02)
Read this article at
Abstract
Aim
VITAL, a phase II single‐arm study, aimed to evaluate efficacy and safety of panitumumab addition to 5‐fluorouracil (5‐FU), mitomycin‐C (MMC) and radiotherapy (RT) in patients with localized squamous cell carcinoma of the anal canal (SCCAC).
Methods
Adult, treatment‐naïve SCCAC patients (Stage T2‐T4, any N, M0) and ECOG‐PS ≤2, received panitumumab (6 mg/kg, day 1 and Q2W; 8 weeks), 5‐FU (1000 mg/m 2/d, days 1‐4 and 29‐32), MMC (10 mg/m 2, days 1 and 29) and RT 45 Gy (1.8 Gy/fraction) to the primary tumor and mesorectal, iliac and inguinal lymph nodes, plus 10‐15 Gy boost dose to the primary tumor and affected lymph nodes. The primary objective was disease free survival rate (DFS) at 3‐years (expected 3‐year DFS rate: 73.7 ± 12%).
Results
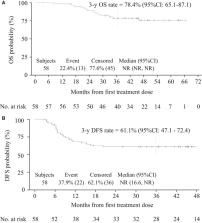
Fifty‐eight patients (31 women; median age: 59 years; ECOG‐PS 0‐1:98%; TNM II [29%] (T2 or T3/N0/M0)/IIIA (T1‐T3/N1/M0 or T4/N0/M0) [21%]/IIIB (T4/N1/M0 or any T/N2 or N3/M0) [47%]/nonevaluable [4%]) were included. The median follow‐up was 45 months. The 3‐year DFS rate was 61.1% (95% CI: 47.1, 72.4). The 3‐year overall survival rate was 78.4% (95% CI: 65.1, 87.1). Eighteen patients (31.0%) required a colostomy within 2 years posttreatment. Grade 3‐4 toxicities were experienced by 53 (91%) patients. Most common grade 3‐4 treatment‐related events were radiation skin injury (40%) and neutropenia (24%). No toxic deaths occurred. Improved efficacy in colostomy‐free survival and complete response rate was observed in human papilloma virus positive patients.
Abstract
VITAL, a phase II single‐arm study, was aimed to evaluate efficacy and safety of panitumumab addition to 5‐fluorouracil (5‐FU), mitomycin‐C (MMC) and radiotherapy in patients with localized squamous cell carcinoma of the anal canal (SCCAC). The study concluded that panitumumab addition to MMC‐5FU regimen in SCCAC patients increases toxicity and does not improve patients’ outcomes.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial
- Record: found
- Abstract: found
- Article: not found
