- Record: found
- Abstract: found
- Article: found
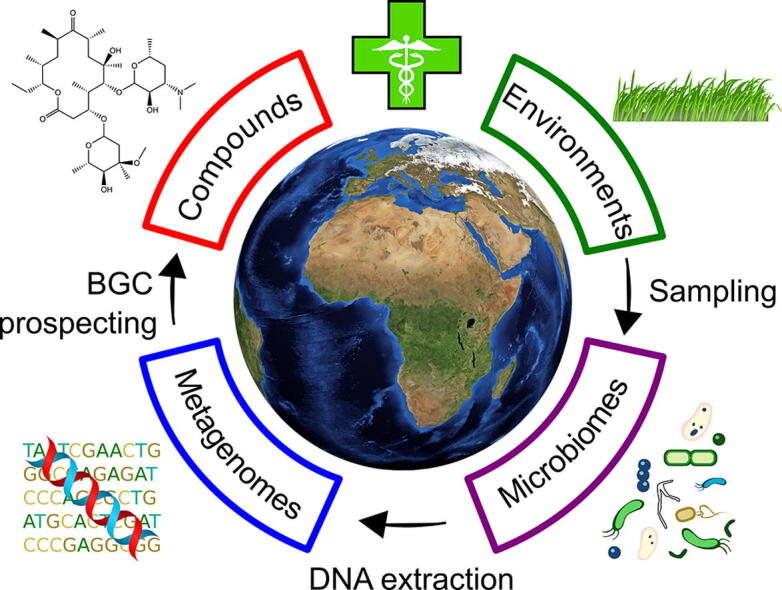
The natural product biosynthesis potential of the microbiomes of Earth – Bioprospecting for novel anti-microbial agents in the meta-omics era
Read this article at
Graphical abstract
Abstract
As we stand on the brink of the post-antibiotic era, we are in dire need of novel antimicrobial compounds. Microorganisms produce a wealth of so-called secondary metabolites and have been our most prolific source of antibiotics so far. However, rediscovery of known antibiotics from well-studied cultured microorganisms, and the fact that the majority of microorganisms in the environment are out of reach by means of conventional cultivation techniques, have led to the exploration of the biosynthetic potential in natural microbial communities by novel approaches. In this mini review we discuss how sequence-based analyses have exposed an unprecedented wealth of potential for secondary metabolite production in soil, marine, and host-associated microbiomes, with a focus on the biosynthesis of non-ribosomal peptides and polyketides. Furthermore, we discuss how the complexity of natural microbiomes and the lack of standardized methodology has complicated comparisons across biomes. Yet, as even the most commonly sampled microbiomes hold promise of providing novel classes of natural products, we lastly discuss the development of approaches applied in the translation of the immense biosynthetic diversity of natural microbiomes to the procurement of novel antibiotics.
Related collections
Most cited references85
- Record: found
- Abstract: found
- Article: not found
Challenges of antibacterial discovery.
- Record: found
- Abstract: found
- Article: not found
A new antibiotic kills pathogens without detectable resistance.
- Record: found
- Abstract: found
- Article: not found