- Record: found
- Abstract: found
- Article: found
Epigenetic repression of miR-17 contributed to di(2-ethylhexyl) phthalate-triggered insulin resistance by targeting Keap1-Nrf2/miR-200a axis in skeletal muscle

Read this article at
Abstract
Rationale: Skeletal muscle insulin resistance is detectable before type 2 diabetes is diagnosed. Exposure to di(2-ethylhexyl) phthalate (DEHP), a typical environmental endocrine-disrupting chemical, is a novel risk factor for insulin resistance and type 2 diabetes. This study aimed to explore insulin signaling regulatory pathway in skeletal muscle of the DEHP-induced insulin-resistant mice and to investigate potential therapeutic strategies for treating insulin resistance.
Methods: C57BL/6J male mice were exposed to 2 mg/kg/day DEHP for 15 weeks. Whole-body glucose homeostasis, oxidative stress and deregulated miRNA-mediated molecular transduction in skeletal muscle were examined. microRNA (miRNA) interventions based on lentiviruses and adeno-associated viruses 9 (AAV9) were performed.
Results: Dnmt3a-dependent promoter methylation and lncRNA Malat1-related sponge functions cooperatively downregulated miR-17 in DEHP-exposed skeletal muscle cells. DEHP suppressed miR-17 to disrupt the Keap1-Nrf2 redox system and to activate oxidative stress-responsive Txnip in skeletal muscle. Oxidative stress upregulated miR-200a, which directly targets the 3'UTR of Insr and Irs1, leading to hindered insulin signaling and impaired insulin-dependent glucose uptake in skeletal muscle, ultimately promoting the development of insulin resistance. AAV9-induced overexpression of miR-17 and lentivirus-mediated silencing of miR-200a in skeletal muscle ameliorated whole-body insulin resistance in DEHP-exposed mice.
Conclusions: The miR-17/Keap1-Nrf2/miR-200a axis contributed to DEHP-induced insulin resistance. miR-17 is a positive regulator, whereas miR-200a is a negative regulator of insulin signaling in skeletal muscle, and both miRNAs have the potential to become therapeutic targets for preventing and treating insulin resistance or type 2 diabetes.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition.
- Record: found
- Abstract: found
- Article: found
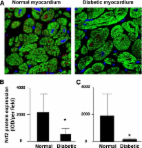
Diabetic Downregulation of Nrf2 Activity via ERK Contributes to Oxidative Stress–Induced Insulin Resistance in Cardiac Cells In Vitro and In Vivo
- Record: found
- Abstract: found
- Article: not found