- Record: found
- Abstract: found
- Article: not found
Differential Responses of Human Regulatory T Cells (Treg) and Effector T Cells to Rapamycin
Read this article at
Abstract
Background
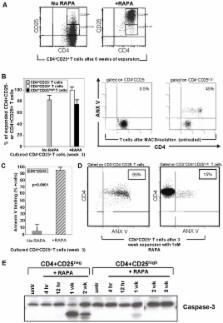
The immunosuppressive drug rapamycin (RAPA) promotes the expansion of CD4 + CD25 highFoxp3 + regulatory T cells via mechanisms that remain unknown. Here, we studied expansion, IL-2R-γ chain signaling, survival pathways and resistance to apoptosis in human Treg responding to RAPA.
Methodology/Principal Findings
CD4 +CD25 + and CD4 +CD25 neg T cells were isolated from PBMC of normal controls (n = 21) using AutoMACS. These T cell subsets were cultured in the presence of anti-CD3/CD28 antibodies and 1000 IU/mL IL-2 for 3 to 6 weeks. RAPA (1–100 nM) was added to half of the cultures. After harvest, the cell phenotype, signaling via the PI3K/mTOR and STAT pathways, expression of survival proteins and Annexin V binding were determined and compared to values obtained with freshly-separated CD4 +CD25 high and CD4 +CD25 neg T cells. Suppressor function was tested in co-cultures with autologous CFSE-labeled CD4 +CD25 neg or CD8 +CD25 neg T-cell responders. The frequency and suppressor activity of Treg were increased after culture of CD4 +CD25 + T cells in the presence of 1–100 nM RAPA (p<0.001). RAPA-expanded Treg were largely CD4 +CD25 highFoxp3 + cells and were resistant to apoptosis, while CD4 +CD25 neg T cells were sensitive. Only Treg upregulated anti-apoptotic and down-regulated pro-apoptotic proteins. Treg expressed higher levels of the PTEN protein than CD4 +CD25 neg cells. Activated Treg±RAPA preferentially phosphorylated STAT5 and STAT3 and did not utilize the PI3K/mTOR pathway.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
A function for interleukin 2 in Foxp3-expressing regulatory T cells.
- Record: found
- Abstract: found
- Article: not found
Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization
- Record: found
- Abstract: found
- Article: not found
