- Record: found
- Abstract: found
- Article: found
Rheumatiod Arthritis: An Updated Overview of Latest Therapy and Drug Delivery
Read this article at
Abstract
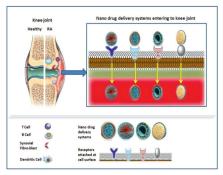
Rheumatoid arthritis is a severe autoimmune disorder, related to joints. It is associated with serious cartilage destruction. This causes disability and reduces the excellence of life. Numerous treatments are existed to combat this disease, however, they are not very efficient and possess severe side effects, higher doses, and frequent administration.
Therefore, newer therapies are developed to overcome all these limitations. These include different monoclonal antibodies, immunoglobulins, small molecules used for immunotherapy and transgenes for gene therapy. One of the main goals of these new generation therapeutics is to address the underlying distressing biological processes by specifically targeting the causative agents with fewer systemic side effects and greater patient console. It is very fortuitous that loads of progressive investigations are going on in this field and many of them have entered into the successful clinical trial. But till date, a limited molecule has got FDA clearance and entered the market for treating this devastating disease.
This review highlights the overview of conventional therapy and advancements in newer therapeutics including immunotherapy and gene therapy for rheumatoid arthritis. Further, different novel techniques for the delivery of these therapeutics of active and passive targeting are also described.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs
- Record: found
- Abstract: found
- Article: not found
Nanoemulsion: Concepts, development and applications in drug delivery.
- Record: found
- Abstract: found
- Article: not found