- Record: found
- Abstract: found
- Article: found
Aggregation-Induced Emission (AIE) Polymeric Micelles for Imaging-Guided Photodynamic Cancer Therapy
Read this article at
Abstract
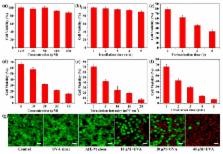
Photodynamic therapy (PDT) is a noninvasive treatment for selectively killing malignant tumor cells. The photosensitizer is a necessary component of photodynamic nanomedicine. Many efforts have been made to develop new photosensitizers for efficient cancer photodynamic therapy. In this work, we report a novel nano photosensitizer, polymeric micelles (AIE-M) with aggregation induced emission characteristic, for photodynamic cancer therapy. AIE-M with sub-20 nm particle size is prepared by the self-assembly of salicylaldazine-incorporated amphiphilic polymer (AIE-1), which can produce reactive oxygen species (ROS) with light irradiation in solution. After uptake by cancer cells, AIE-M can specially sojourn in plasma membranes of cancer cells at the early stage and predominantly accumulate in the mitochondria of cancer cell at the late stage. The phototoxicity of AIE-M, resulting from the generation of intracellular ROS with light irradiation, can efficiently cause cancer cells death by apoptosis and necrosis. The advantages of AIE-M as a nano photosensitizer include the small size, highly colloidal stability in the process of preparation and storage, and high cell penetration. The ultra-low Critical Micelle Concentration (CMC) of AIE-1, negligible dark toxicity and super phototoxicity of AIE-M suggest its promising potential for image-guided PDT.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Cancer Cell-Selective In Vivo Near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules

- Record: found
- Abstract: found
- Article: found
Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions
- Record: found
- Abstract: not found
- Article: not found