- Record: found
- Abstract: found
- Article: found
Clinical Outcomes, Costs, and Cost-effectiveness of Strategies for Adults Experiencing Sheltered Homelessness During the COVID-19 Pandemic
Key Points
Question
What are the projected clinical outcomes and costs associated with strategies for reducing severe acute respiratory syndrome coronavirus 2 infections among people experiencing sheltered homelessness?
Findings
In this decision analytic model, daily symptom screening with polymerase chain reaction (PCR) testing of individuals who had positive symptom screening paired with nonhospital care site management of people with mild to moderate coronavirus disease 2019 (COVID-19) was associated with a substantial decrease in infections and lowered costs over 4 months compared with no intervention across a wide range of epidemic scenarios. In a surging epidemic, adding periodic universal PCR testing to symptom screening and nonhospital care site management was associated with improved clinical outcomes at modestly increased costs.
Meaning
In this study, daily symptom screening with PCR testing of individuals who had positive symptom screening and use of alternative care sites for COVID-19 management among individuals experiencing sheltered homelessness were associated with substantially reduced new cases and costs compared with other strategies.
Abstract
This decision analytic model assesses the clinical outcomes, costs, and cost-effectiveness associated with strategies for coronavirus disease 2019 (COVID-19) among US adults experiencing sheltered homelessness.
Abstract
Importance
Approximately 356 000 people stay in homeless shelters nightly in the United States. They have high risk of contracting coronavirus disease 2019 (COVID-19).
Objective
To assess the estimated clinical outcomes, costs, and cost-effectiveness associated with strategies for COVID-19 management among adults experiencing sheltered homelessness.
Design, Setting, and Participants
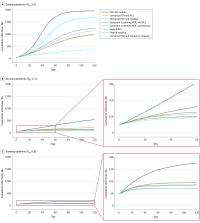
This decision analytic model used a simulated cohort of 2258 adults residing in homeless shelters in Boston, Massachusetts. Cohort characteristics and costs were adapted from Boston Health Care for the Homeless Program. Disease progression, transmission, and outcomes data were taken from published literature and national databases. Surging, growing, and slowing epidemics (effective reproduction numbers [R e], 2.6, 1.3, and 0.9, respectively) were examined. Costs were from a health care sector perspective, and the time horizon was 4 months, from April to August 2020.
Exposures
Daily symptom screening with polymerase chain reaction (PCR) testing of individuals with positive symptom screening results, universal PCR testing every 2 weeks, hospital-based COVID-19 care, alternative care sites (ACSs) for mild or moderate COVID-19, and temporary housing were each compared with no intervention.
Main Outcomes and Measures
Cumulative infections and hospital-days, costs to the health care sector (US dollars), and cost-effectiveness, as incremental cost per case of COVID-19 prevented.
Results
The simulated population of 2258 sheltered homeless adults had a mean (SD) age of 42.6 (9.04) years. Compared with no intervention, daily symptom screening with ACSs for pending tests or confirmed COVID-19 and mild or moderate disease was associated with 37% fewer infections (1954 vs 1239) and 46% lower costs ($6.10 million vs $3.27 million) at an R e of 2.6, 75% fewer infections (538 vs 137) and 72% lower costs ($1.46 million vs $0.41 million) at an R e of 1.3, and 51% fewer infections (174 vs 85) and 51% lower costs ($0.54 million vs $0.26 million) at an R e of 0.9. Adding PCR testing every 2 weeks was associated with a further decrease in infections; incremental cost per case prevented was $1000 at an R e of 2.6, $27 000 at an R e of 1.3, and $71 000 at an R e of 0.9. Temporary housing with PCR every 2 weeks was most effective but substantially more expensive than other options. Compared with no intervention, temporary housing with PCR every 2 weeks was associated with 81% fewer infections (376) and 542% higher costs ($39.12 million) at an R e of 2.6, 82% fewer infections (95) and 2568% higher costs ($38.97 million) at an R e of 1.3, and 59% fewer infections (71) and 7114% higher costs ($38.94 million) at an R e of 0.9. Results were sensitive to cost and sensitivity of PCR and ACS efficacy in preventing transmission.
Conclusions and Relevance
In this modeling study of simulated adults living in homeless shelters, daily symptom screening and ACSs were associated with fewer severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and decreased costs compared with no intervention. In a modeled surging epidemic, adding universal PCR testing every 2 weeks was associated with further decrease in SARS-CoV-2 infections at modest incremental cost and should be considered during future surges.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China
- Record: found
- Abstract: found
- Article: not found