- Record: found
- Abstract: found
- Article: found
Alendronate as an Effective Treatment for Bone Loss and Vascular Calcification in Kidney Transplant Recipients
Read this article at
Abstract
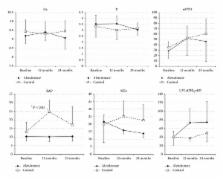
Kidney transplant recipients develop secondary osteoporosis induced by immunosuppressive medication, with a high risk of fracture, and abdominal aortic calcification (AC) is a known predictor of cardiovascular mortality. In this study of 12 stable kidney recipients, we estimated the preventive effect of bisphosphonate treatment on bone loss and progression of AC. We randomly divided the subjects into a treatment group with alendronate (group A: 5 subjects) and a control group (group C: 7 subjects). Group A patients received 35 mg/week of alendronate over 24 months, while group C patients were not administered with any bisphosphonates. Two major endpoints were established: (1) the time-dependent change in bone mineral density (BMD) estimated with DEXA and (2) progression of abdominal AC, calculated twice as an index (ACI) using computed tomography data. Over the 2-year study period, group A patients showed significantly increased BMD of 1.86 ± 0.85% ( P = 0.015 versus baseline), and almost complete inhibition of ACI progression (38.2 ± 24.2% to 39.6 ± 24.3%), but group C patients showed a decrease in BMD decline with bone loss and progression of ACI (32.8 ± 25.0% to 37.8 ± 29.2%, P = 0.061). In conclusion, alendronate therapy was an effective treatment in kidney transplant recipients for secondary osteoporosis and vascular calcification as ectopic calcification. This clinical trial is registered with number JMA-IIA00155 of JMACCT CTR.
Related collections
Most cited references59
- Record: found
- Abstract: not found
- Article: not found
Clinical epidemiology of cardiovascular disease in chronic renal disease.
- Record: found
- Abstract: found
- Article: not found
Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group.
- Record: found
- Abstract: found
- Article: not found