- Record: found
- Abstract: found
- Article: found
A cell-active cyclic peptide targeting the Nrf2/Keap1 protein–protein interaction†
Read this article at
Abstract
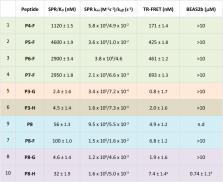
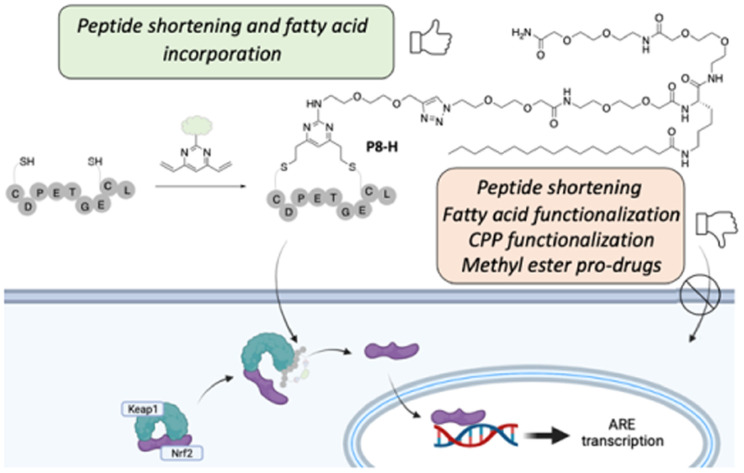
The disruption of the protein–protein interaction (PPI) between Nrf2 and Keap1 is an attractive strategy to counteract the oxidative stress that characterises a variety of severe diseases. Peptides represent a complementary approach to small molecules for the inhibition of this therapeutically important PPI. However, due to their polar nature and the negative net charge required for binding to Keap1, the peptides reported to date exhibit either mid-micromolar activity or are inactive in cells. Herein, we present a two-component peptide stapling strategy to rapidly access a variety of constrained and functionalised peptides that target the Nrf2/Keap1 PPI. The most promising peptide, P8-H containing a fatty acid tag, binds to Keap1 with nanomolar affinity and is effective at inducing transcription of ARE genes in a human lung epithelial cell line at sub-micromolar concentration. Furthermore, crystallography of the peptide in complex with Keap1 yielded a high resolution X-ray structure, adding to the toolbox of structures available to develop cell-permeable peptidomimetic inhibitors.
Abstract
Application of DVP stapling methodology to efficiently develop Nrf2 peptides with nM activity in enzymatic assays, submicromolar activity in cells and non-cytotoxicity, characterised by high resolution X-ray co-crystallography.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Monoacidic Inhibitors of the Kelch-like ECH-Associated Protein 1: Nuclear Factor Erythroid 2-Related Factor 2 (KEAP1:NRF2) Protein-Protein Interaction with High Cell Potency Identified by Fragment-Based Discovery.
- Record: found
- Abstract: found
- Article: not found