- Record: found
- Abstract: found
- Article: found
Icariin-loaded electrospun PCL/gelatin sub-microfiber mat for preventing epidural adhesions after laminectomy
Abstract
Background
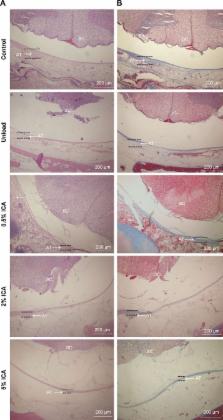
Epidural adhesion is one of the major reasons attributed to failed back surgery syndrome after a successful laminectomy, and results in serious clinical complications which require management from physicians. Therefore, there is an urgent demand within the field to develop biodegradable anti-adhesion membranes for the prevention of post-operative adhesion.
Methods
In this study, icariin (ICA) was initially loaded into polycaprolactone (PCL)/gelatin fibers via electrospinning to fabricate nanofibrous membranes. The effects of the ICA content (0.5wt%, 2wt% and 5wt%) and the bioactivity of ICA in the nanofibrous membranes were investigated in vitro and in vivo.
Results
The nanofibrous membranes showed suitable pore size and good properties that were unaffected by ICA concentration. Moreover, the ICA-loaded membranes exhibited an originally rapid and subsequently gradual sustained ICA release profile that could significantly prevent fibroblast adhesion and proliferation. In vivo studies with rabbit laminectomy models demonstrated that the ICA-loaded membranes effectively reduced epidural adhesion by gross observation, histology, and biochemical evaluation. The anti-adhesion mechanism of ICA was found to be via suppression of the TGF-β/Smad signaling proteins and down regulation of collage I/III and a-SMA expression for the first time.
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Biomimetic electrospun nanofibrous structures for tissue engineering.
- Record: found
- Abstract: found
- Article: not found
Biodegradable electrospun fibers for drug delivery.
- Record: found
- Abstract: found
- Article: not found