- Record: found
- Abstract: found
- Article: found
Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors

Read this article at
Abstract
Nestor et al. examine how lupus antibodies that enter the brain cause neuronal dysfunction and cognitive impairment. The results show that activated microglia are critical for neuronal damage and that inhibiting them can preserve neuronal function and cognition.
Abstract
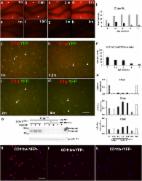
Cognitive impairment occurs in 40–90% of patients with systemic lupus erythematosus (SLE), which is characterized by autoantibodies to nuclear antigens, especially DNA. We discovered that a subset of anti-DNA antibodies, termed DNRAbs, cross reacts with the N-methyl- d-aspartate receptor (NMDAR) and enhances NMDAR signaling. In patients, DNRAb presence associates with spatial memory impairment. In a mouse model, DNRAb-mediated brain pathology proceeds through an acute phase of excitotoxic neuron loss, followed by persistent alteration in neuronal integrity and spatial memory impairment. The latter pathology becomes evident only after DNRAbs are no longer detectable in the brain. Here we investigate the mechanism of long-term neuronal dysfunction mediated by transient exposure to antibody. We show that activated microglia and C1q are critical mediators of neuronal damage. We further show that centrally acting inhibitors of angiotensin-converting enzyme (ACE) can prevent microglial activation and preserve neuronal function and cognitive performance. Thus, ACE inhibition represents a strong candidate for clinical trials aimed at mitigating cognitive dysfunction.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Novel role of PKR in inflammasome activation and HMGB1 release
- Record: found
- Abstract: found
- Article: found
Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain
- Record: found
- Abstract: found
- Article: not found