- Record: found
- Abstract: found
- Article: not found
Hydrogen peroxide reactivity and specificity in thiol-based cell signalling
Abstract
Reversible oxidation of thiol proteins is an important cell signalling mechanism. In many cases, this involves generation or exposure of the cells to H2O2, and oxidation of proteins that are not particularly H2O2-reactive. There is a conundrum as to how these proteins are oxidised when other highly reactive proteins such as peroxiredoxins are present. This article discusses potential mechanisms, focussing on recent evidence for oxidation being localised within the cell, redox relays involving peroxiredoxins operating in some signalling pathways, and mechanisms for facilitated or directed oxidation of specific targets. These findings help define conditions that enable redox signalling but there is still much to learn regarding mechanisms.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis.
- Record: found
- Abstract: found
- Article: not found
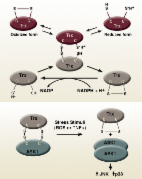
Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1.
- Record: found
- Abstract: found
- Article: found