- Record: found
- Abstract: found
- Article: found
Modulation of Breast Cancer Cell FASN Expression by Obesity-Related Systemic Factors

Read this article at
Abstract
Purpose:
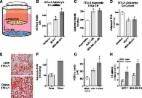
The objective of this study is to determine the impact of exposure to obesity-related systemic factors on fatty acid synthase enzyme (FASN) expression in breast cancer cells.
Methods:
MCF-7 breast cancer cells were exposed to sera from patients having obesity or not having obesity and subjected to quantitative reverse transcription polymerase chain reaction (RT-qPCR). Subsequent MTT and colony-forming assays using both MCF-7 and T-47D cells exposed to sera and treated with or without FASN inhibitor, TVB-3166, were used. MCF-7 cells were then treated with insulin and the sterol regulatory element–binding protein (SREBP) processing inhibitor, betulin, prior to analysis of FASN expression by quantitative RT-qPCR and western blot. Insulin-induced SREBP-FASN promoter binding was analyzed by chromatin immunoprecipitation with an anti-SREBP antibody.
Results:
In response to sera exposure (body mass index [BMI] >30) there was an increase in FASN expression in breast cancer cells. Furthermore, treatment with the FASN inhibitor, TVB-3166, resulted in a decreased breast cancer cell survival and proliferation while increasing apoptosis upon sera exposure (BMI >30). Insulin-exposed MCF-7 cells exhibited an increased FASN messenger RNA and protein expression, which is abrogated upon SREBP inhibition. In addition, insulin exposure induced enhanced SREBP binding to the FASN promoter.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: found
Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis.
- Record: found
- Abstract: found
- Article: not found