- Record: found
- Abstract: found
- Article: found
COVID-19 and Acute Sarcopenia
Read this article at
Abstract
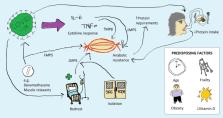
The COVID-19 pandemic has had a devastating global impact, with older adults being most at risk of death from the disease. However, acute sarcopenia occurs in survivors of COVID-19; older adults and the most critically unwell patients are the most at risk. Acute sarcopenia is an under-recognised condition of acute muscle insufficiency, defined by declines in muscle function and/or quantity within six months, usually following a stressor event. This commentary reviews definition and mechanisms of acute sarcopenia in COVID-19 and suggests recommendations for research and clinical practice. Research should now focus on the longer-term consequences of acute sarcopenia in patients who have suffered from COVID-19. At the same time, clinicians need to be increasingly aware of the condition, and measurements of muscle strength, quantity, and physical performance should be embedded into clinical practice. Clinicians should consider the risks of acute sarcopenia when weighing up the risks and benefits of treatment (e.g. dexamethasone), and instigate multidisciplinary treatment including dietetics input.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report

- Record: found
- Abstract: found
- Article: found