- Record: found
- Abstract: found
- Article: found
Diversity and Spatial Structure of Belowground Plant–Fungal Symbiosis in a Mixed Subtropical Forest of Ectomycorrhizal and Arbuscular Mycorrhizal Plants
Read this article at
Abstract
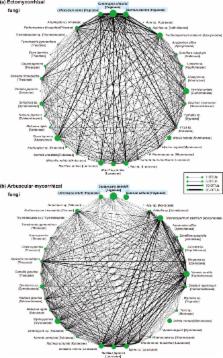
Plant–mycorrhizal fungal interactions are ubiquitous in forest ecosystems. While ectomycorrhizal plants and their fungi generally dominate temperate forests, arbuscular mycorrhizal symbiosis is common in the tropics. In subtropical regions, however, ectomycorrhizal and arbuscular mycorrhizal plants co-occur at comparable abundances in single forests, presumably generating complex community structures of root-associated fungi. To reveal root-associated fungal community structure in a mixed forest of ectomycorrhizal and arbuscular mycorrhizal plants, we conducted a massively-parallel pyrosequencing analysis, targeting fungi in the roots of 36 plant species that co-occur in a subtropical forest. In total, 580 fungal operational taxonomic units were detected, of which 132 and 58 were probably ectomycorrhizal and arbuscular mycorrhizal, respectively. As expected, the composition of fungal symbionts differed between fagaceous (ectomycorrhizal) and non-fagaceous (possibly arbuscular mycorrhizal) plants. However, non-fagaceous plants were associated with not only arbuscular mycorrhizal fungi but also several clades of ectomycorrhizal (e.g., Russula) and root-endophytic ascomycete fungi. Many of the ectomycorrhizal and root-endophytic fungi were detected from both fagaceous and non-fagaceous plants in the community. Interestingly, ectomycorrhizal and arbuscular mycorrhizal fungi were concurrently detected from tiny root fragments of non-fagaceous plants. The plant–fungal associations in the forest were spatially structured, and non-fagaceous plant roots hosted ectomycorrhizal fungi more often in the proximity of ectomycorrhizal plant roots. Overall, this study suggests that belowground plant–fungal symbiosis in subtropical forests is complex in that it includes “non-typical” plant–fungal combinations (e.g., ectomycorrhizal fungi on possibly arbuscular mycorrhizal plants) that do not fall within the conventional classification of mycorrhizal symbioses, and in that associations with multiple functional (or phylogenetic) groups of fungi are ubiquitous among plants. Moreover, ectomycorrhizal fungal symbionts of fagaceous plants may “invade” the roots of neighboring non-fagaceous plants, potentially influencing the interactions between non-fagaceous plants and their arbuscular-mycorrhizal fungal symbionts at a fine spatial scale.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Phylogenetic distribution and evolution of mycorrhizas in land plants.
- Record: found
- Abstract: found
- Article: not found
Large-scale forest girdling shows that current photosynthesis drives soil respiration.
- Record: found
- Abstract: found
- Article: not found