- Record: found
- Abstract: found
- Article: not found
Cost Effectiveness of Guanfacine Extended Release as an Adjunctive Therapy to a Stimulant Compared with Stimulant Monotherapy for the Treatment of Attention-Deficit Hyperactivity Disorder in Children and Adolescents
Read this article at
Abstract
Background
Attention-deficit hyperactivity disorder (ADHD) is a common psychiatric disorder in childhood, affecting 3–7% of school-age children in the US and imposing substantial economic burden. Stimulants are considered first-line pharmacological treatment and are the most prescribed treatment for ADHD. However, approximately 30% of children with ADHD do not have an optimal response to a single stimulant and may require adjunctive therapy.
Objective
Our objective was to conduct a cost-effectiveness analysis (CEA) of adding a non-stimulant, guanfacine extended release (GXR), to stimulants versus maintaining existing stimulant monotherapy in the treatment of ADHD in children and adolescents with suboptimal response to stimulant monotherapy.
Methods
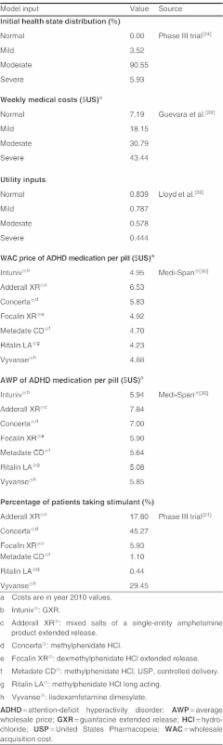
A 1-year Markov model was developed to estimate costs and effectiveness from a US third-party payer perspective. Effectiveness was measured by the QALY. The model assumed that patients transitioned among four health states (normal, mild, moderate and severe), defined by the Clinical Global Impression-Severity (CGI-S) scale. Transition probabilities were estimated in an ordered logit model using patient-level data from a multicentre, 9-week, double-blind, placebo-controlled, dose-optimization study, where subjects (n=461) continued their stable morning stimulant and were randomized to GXR administered in the morning, GXR administered in the evening, or placebo. The model assumed that patients in moderate/severe health states after week 8 would discontinue ADHD treatment and remain in that state for the rest of the study period. Direct costs included drug wholesale acquisition costs and health state costs, all in $US, year 2010 values. Utility associated with each health state was obtained from the literature and disutilities associated with adverse events were applied for the first 4 weeks. Oneway sensitivity analyses and probabilistic sensitivity analysis (PSA) were conducted by varying costs, utilities, adverse-event duration, and transition probabilities.
Results
Compared with maintaining existing stimulant monotherapy, adding GXR to existing stimulant monotherapy was associated with an incremental drug cost of $US1016 but a lower medical cost of $US124, resulting in a total incremental cost of $US892 at 1 year. The addition of GXR to stimulants led to an incremental QALY of 0.03 and an incremental cost-effectiveness ratio (ICER) of $US31 660/QALY. In one-way sensitivity analysis, ICER values ranged from $US19 723, when 100% of patients were assumed to be severe in their initial health state, to $US46631, when the last observed states from the clinical trial were carried forward to the end of the 1-year analysis period. PSA demonstrated a 94.6% likelihood that the ICER falls below $US50 000/QALY.
Conclusions
The impairment associated with residual ADHD symptoms after stimulant therapy is becoming increasingly recognized. This is the first analysis of the cost effectiveness of stimulants combined with an adjunctive medication. This study suggests that the adjunctive therapy of GXR with stimulants is a cost-effective treatment based on a willingness-to-pay threshold of $US50 000/QALY. This may address an unmet need among patients with suboptimal response to stimulant monotherapy.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment.
- Record: found
- Abstract: found
- Article: not found
Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life.
- Record: found
- Abstract: found
- Article: not found
